Protein Trafficking is a Major Alzheimer Disease Pathway
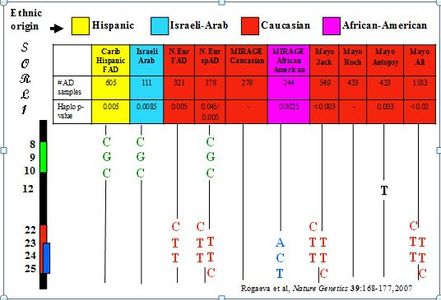
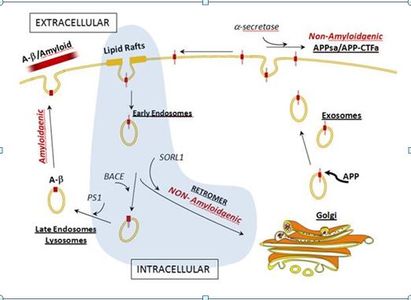
The accumulation of amyloid β-peptide (Aβ) is widely recognized as central to the pathogenesis of Alzheimer disease (AD). Endocytosis of amyloid precursor protein (APP) and its subsequent cleavage by BACE and PS1 are primary events in the production of Aβ. However, while much is known about the proteolytic events involved in APP processing and formation of Aβ, factors that control the trafficking and processing of APP and their role in AD are poorly understood. We postulated that processing of APP to generate non-toxic forms is mediated by mechanisms of membrane trafficking within the endocytic system that direct APP away from BACE by returning APP to the Golgi. A key component of the endosome-to-Golgi pathway is the retromer complex. Initially, we identified SORL1 among a class of neuronal sorting receptors containing a Vps10 domain (sortilins) as a key player in enhancing retromer retrieval and non-amyloidogenic processing of APP.1 This association was subsequently confirmed in large genome-wide association studies.2,3 In addition, we showed for the first time association of AD with SORCS1, SORCS2, SORCS3 and SORT1.4,5
Based on these findings, we hypothesized that variants in other genes in endocytosis and retromer sorting pathways might also modulate AD risk. We tested genetic association of AD with 451 polymorphisms in 15 genes encoding retromer or retromer-associated proteins in a Caucasian sample of 8,309 AD cases and 7,366 cog nitively normal elders using individual SNP and gene-based tests.6 We obtained significant evidence of association with KIAA1033 (p = 0.025), SNX1 (p =0.035), SNX3 (p = 0.0057) and RAB7A (p = 0.018). Ten KIAA1033 SNPs were also significantly associated with AD in a group of African Americans (513 AD cases, 504 controls). Findings with four significant SNX3 SNPs in the discovery sample were replicated in a community-based sample of Israeli-Arabs (124 AD cases, 142 controls). We also showed that Snx3 and Rab7A proteins interact with the cargo-selective retromer complex through independent mechanisms to regulate the membrane association of retromer and thereby are key mediators of retromer function. These data implicate additional AD risk genes in the retromer pathway and formally demonstrate a direct link between the activity of the retromer complex and the pathogenesis of AD.
- Rogaeva E, Meng Y, Lee JH, Gu Y-J, Zou F, Kawarai T, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, T.Cuenco K, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland R, Inzelberg R, Hampe W, Bujo H, Song Y, Andersen O, Graff-Radford N, Petersen R, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The sortilin-related receptor SORL1 is functionally and genetically associated with Alzheimer’s disease. Nat Genet 2007; 39:168-177. PMID: 17220890 http://www.nature.com/ng/journal/v39/n2/full/ng1943.html
- Miyashita A, Koike A, Jun G, …54 co-authors…, Schellenberg GD, Farrer LA, Kuwano R. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS ONE 2013; 8(4): e58618. PMID: 23565137 http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0058618
- Lambert J-C, Ibrahim-Verbaas CA, Harold D, …163 co-authors…, Farrer LA, van Duijn CM, Van Broekhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P. Extended meta-analysis of 74,538 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 2013; 45:1452-1458. PMID: 24162737 http://www.nature.com/ng/journal/v45/n12/full/ng.2802.html
- Reitz C, Tokuhiro S, Clark LN, Conrad C, Vonsattel J-P, Palotas A, Lantigua R, Medrano M, Jiménez-Velázquez IZ, Haines JL,Pericak-Vance MA, Farrer LA, Lee JH, Rogaeva E, St. George-Hyslop P, Mayeux R. SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer’s disease risk. Ann Neurol 2011; 69:47-64. PMID: 21280075 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3086759/
- Reitz C, Tosto G, Vardarajan B, Rogaeva E, Ghani M, Rogers RS, Conrad C, Haines JL, Pericak-Vance MA, Fallin MD, Foroud T, Farrer LA, Schellenberg GD, George-Hyslop PS, Mayeux R. Independent and epistatic effects of variants in VPS10-d receptors on Alzheimer disease risk and processing of the amyloid precursor protein (APP). Transl Psychiatry 2013;3:e256. PMID: 2367346. http://www.nature.com/tp/journal/v3/n5/pdf/tp201313a.pdf
- Vardarajan BN, Bruesegem SY, Harbour ME, Inzelberg R, Friedland R, St. George-Hyslop P, Seaman MNJ, Farrer LA. Identification of Alzheimer disease associated variants in genes that regulate retromer function. Neurobiol Aging 2012; 33:2231.e15-2231.e30 PMID: 22673115 http://www.sciencedirect.com/science/article/pii/S0197458012002710