The Zaia Lab, in collaboration with Prof. Joanna Phillips of the Dept. of Nerological Surgery at the University of California San Francisco, has a new publication out in Molecular & Cellular Proteomics, “In-depth matrisome and glycoproteomic analysis of human brain glioblastoma versus control tissue.”
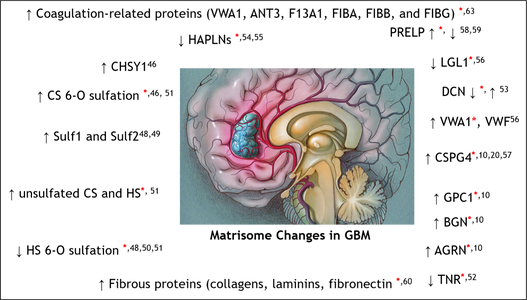
Glioblastoma (GBM) is the most common and malignant primary brain tumor. The extracellular matrix (ECM), also known as the matrisome, helps determine glioma invasion, adhesion, and growth. Little attention, however, has been paid to glycosylation of the ECM components that constitute the majority of glycosylated protein mass and presumed biological properties. To acquire a comprehensive understanding of the biological functions of the matrisome and its components, including proteoglycans and glycosaminoglycans (GAGs), in GBM tumorigenesis, and to identify potential biomarker candidates, we studied the alterations of GAGs, including heparan sulfate (HS) and chondroitin sulfate (CS), the core proteins of proteoglycans, and other glycosylated matrisomal proteins in GBM subtypes vs. control human brain tissue samples. We scrutinized the proteomics data to acquire in-depth site-specific glycoproteomic profiles of the GBM subtypes that will assist in identifying specific glycosylation changes in GBM. We observed an increase in CS 6-O sulfation and a decrease in HS 6-O sulfation, accompanied by an increase in unsulfated CS and HS disaccharides in GBM vs. control samples. Several core matrisome proteins, including proteoglycans (decorin, biglycan, agrin, prolargin, glypican-1, CSPG4), tenascin, fibronectin, hyaluronan link protein 1 and 2, laminins, and collagens, were differentially regulated in GBM vs. controls.
Interestingly, a higher degree of collagen hydroxyprolination was also observed for GBM vs. controls. Further, two proteoglycans, CSPG4, and agrin were significantly lower, about 6–fold for IDH-mutant, compared to the WT GBM samples. Differential regulation of O-glycopeptides for proteoglycans, including brevican, neurocan, and versican, was observed for GBM subtypes vs. controls. Moreover, an increase in levels of glycosyltransferase and glycosidase enzymes was observed for GBM when compared to control samples. We also report distinct protein, peptide, and glycopeptide features for GBM subtypes comparisons. Taken together, our study informs understanding of the alterations to key matrisomal molecules that occur during GBM development.
Corresponding author- Prof. Joseph Zaia, Dept. of Biochemistry, Center for Biomedical Mass Spectrometry, Boston University.
Collaborator- Prof. Joanna Phillips, Dept. of Neurological Surgery, Brain Tumor Center, Helen Diller Family Cancer Research Center, University of California San Francisco, Division of Neuropathology, Department of Pathology, University of California San Francisco.