A new study from the Layne laboratory: “Mechanisms of aortic carboxypeptidase-like protein secretion and identification of an intracellularly retained variant associated with Ehlers–Danlos syndrome” was published in The Journal of Biological Chemistry. This study identified that a defective form of the secreted protein aortic carboxypeptidase-like protein (ACLP) from patients with Ehlers-Danlos syndrome (EDS) is retained in cells and induces cellular stress. This collaborative study was with the Wong and Smith labs from the BU College of Engineering. 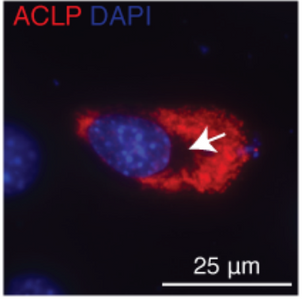
EDS is a prevalent genetic disease that results in weakened connective tissues. This disease results in joint hypermobility, vascular disruption, and aberrant wound healing. Mutations in the gene that encodes for ACLP have recently been identified to cause a novel variant of EDS. Furthermore, excessive amounts of ACLP cause fibrosis in multiple organs including the lung, liver, and adipose tissue.
Detailed characterization of a mutant form of ACLP identified in an EDS patient revealed that this protein was retained in cells. Collagen fibers were generated in vitro that contained ACLP and compared to collagen only controls, they found that fibers with ACLP exhibited increased mechanical properties consistent with a function in maintaining connective tissue integrity. This study was also the first to show that ACLP contributes to the mechanical strength of collagen fibers that make up numerous connective tissues including ligaments, tendons, and cartilage.