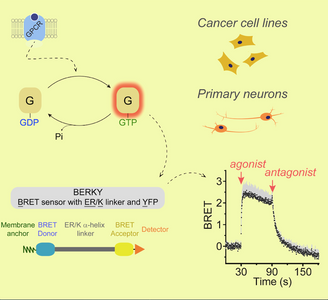
Research News: New Discovery from the Garcia-Marcos Laboratory
The Garcia-Marcos laboratory has discovered that heterotrimeric G-proteins, which are critical molecular switches in cellular communication processes, can trigger different responses in cells depending on the type of protein that activates them.
Heterotrimeric G-proteins are typically activated by G-protein-coupled receptors (GPCRs), which are the target for over one-third of drugs approved for use in the clinic. But G-proteins can also be activated by other proteins that are not GPCRs, a mechanism with important implications for human physiology and disease. The Garcia-Marcos laboratory has used an innovative approach to dissect and compare the consequences of G-protein stimulation by different activators, including GPCRs and various non-GPCR proteins. The approach leveraged a recently developed type of biosensors capable of detecting different forms of active G-proteins in living cells in real time. These were combined with engineered G-protein activator constructs that could be turned on at will with an exogenous synthetic chemical.
The main conclusion is that, contrary to previous beliefs, GPCR and non-GPCR activators elicit different forms of G-protein activation in cells. These findings have important implications in our understanding of pharmacologically actionable signaling hubs in cells, which could be leveraged to envision and design new therapeutic agents.
You can read the article here:
Garcia-Marcos M. Complementary biosensors reveal different G-protein signaling modes triggered by GPCRs and non-receptor activators. Elife. 2021 Mar 31;10:e65620. PMID: 33787494 https://pubmed.ncbi.nlm.nih.gov/33787494/
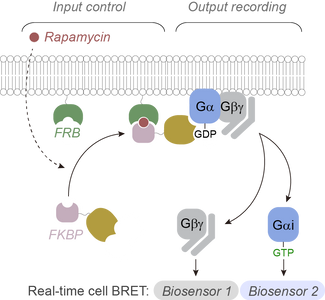
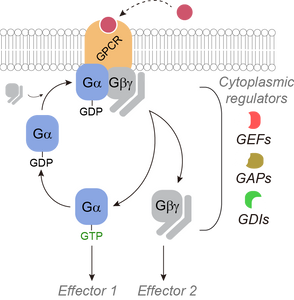
Research discoveries: seeing G-protein signaling
New research discoveries: Regulators of G protein signaling in cancer
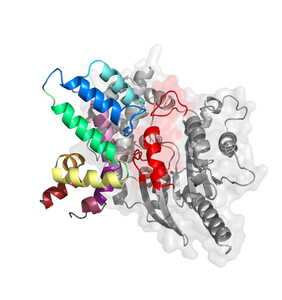 The Garcia-Marcos laboratory has recently published a new study in the journal Science Signaling. In this study, the authors characterized how cancer-associated mutations in a family of negative regulators of G proteins affect the ability of these regulators to modulate G protein activity. Many of these mutations turn out to be deleterious for the G protein regulatory function. In the words of the journal's Editor:
The Garcia-Marcos laboratory has recently published a new study in the journal Science Signaling. In this study, the authors characterized how cancer-associated mutations in a family of negative regulators of G proteins affect the ability of these regulators to modulate G protein activity. Many of these mutations turn out to be deleterious for the G protein regulatory function. In the words of the journal's Editor:
"Mutations in the genes encoding the α subunits of heterotrimeric G proteins are associated with cancer. In particular, mutations that prevent the Gα subunits from hydrolyzing GTP, thus rendering them constitutively active, are pro-oncogenic. DiGiacomo et al. surveyed cancer-associated mutations in regulator of G protein signaling (RGS) proteins, which are physiological inhibitors of G proteins. Through bioinformatics analysis, genetic interaction studies in yeast, and functional assays in mammalian cells, the authors showed that many cancer-associated RGS mutants fail to inhibit G protein signaling because of reduced protein stability or impaired interactions with their targets. With these tools, further cancer-associated mutations in RGS proteins can be characterized."
This work was spearheaded by the former Garcia-Marcos' laboratory postdoc Vincent DiGiacomo, who is currently working in the Cambridge biotech company DeepBiome, with the help of other laboratory members, including two undergraduate students from Boston University. The entire study was carried out in the Department of Biochemistry.
Research Discoveries: New ways of activating G-proteins during embryonic development
Heterotrimeric G proteins are signaling switches that control cellular communication 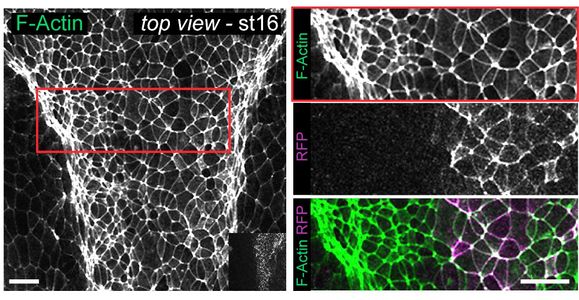 across metazoans. From a traditional standpoint, these G-proteins are activated by G-protein-coupled receptors (GPCRs). However, a recent paper published in the Journal of Cell Biology by Arthur Marivin and colleagues provides direct evidence that heterotrimeric G-proteins can be activated in vivo by a cytoplasmic factor instead of by a GPCR. Specifically, DAPLE, a non-receptor protein bearing an evolutionarily conserved G-protein activating motif, triggers apical cell constriction during neurulation in Xenopus and zebrafish embryos via G-protein dependent signaling. This project of the Garcia-Marcos Lab was carried out in collaboration with the Dominguez Lab (Dept. of Medicine) and the Cifuentes Lab (Dept. of Biochemistry) at BU.
across metazoans. From a traditional standpoint, these G-proteins are activated by G-protein-coupled receptors (GPCRs). However, a recent paper published in the Journal of Cell Biology by Arthur Marivin and colleagues provides direct evidence that heterotrimeric G-proteins can be activated in vivo by a cytoplasmic factor instead of by a GPCR. Specifically, DAPLE, a non-receptor protein bearing an evolutionarily conserved G-protein activating motif, triggers apical cell constriction during neurulation in Xenopus and zebrafish embryos via G-protein dependent signaling. This project of the Garcia-Marcos Lab was carried out in collaboration with the Dominguez Lab (Dept. of Medicine) and the Cifuentes Lab (Dept. of Biochemistry) at BU.
G-protein discoveries
The Garcia-Marcos lab has recently published two studies in the Journal of Biological  Chemistry on the topic of heterotrimeric G protein signaling. Marcin Maziarz, a postdoctoral fellow in the Garcia-Marcos lab, is the first author in both of them. The first study, which was selected as an Editors’ Pick, describes a novel pipeline for the discovery and validation of G protein activators that are not GPCRs, which are the “classic” G protein activators. By screening candidate cytoplasmic proteins containing a putative Gα-binding-and-activating (GBA) motif and using various in vitro and cell-based assays, the lab identified PLCδ4b as a new non-receptor G protein activator.
Chemistry on the topic of heterotrimeric G protein signaling. Marcin Maziarz, a postdoctoral fellow in the Garcia-Marcos lab, is the first author in both of them. The first study, which was selected as an Editors’ Pick, describes a novel pipeline for the discovery and validation of G protein activators that are not GPCRs, which are the “classic” G protein activators. By screening candidate cytoplasmic proteins containing a putative Gα-binding-and-activating (GBA) motif and using various in vitro and cell-based assays, the lab identified PLCδ4b as a new non-receptor G protein activator.
In the second study, the lab interrogated the mechanism of action of mutant G proteins known to drive uveal melanoma, a cancer of the eye which lacks effective therapies. Interestingly, they found that one frequent mutation, Q209P in the G protein Gαq, leads to G protein activation through a unique and unanticipated mechanism that could be leveraged to develop novel therapeutics for this cancer type.
Mikel Garcia-Marcos Promotion
The Department would like to congratulate Dr. Mikel Garcia-Marcos on his recent promotion to Associate Professor of Biochemistry. This is a well-deserved honor!
Inhibiting G protein signaling and disease
Citation: Specific inhibition of GPCR-independent G protein signaling by a rationally engineered protein.
Leyme A, Marivin A, Maziarz M, DiGiacomo V, Papakonstantinou MP, Patel PP, Blanco-Canosa JB, Walawalkar IA, Rodriguez-Davila G, Dominguez I, Garcia-Marcos M. Proc Natl Acad Sci U S A. 2017 Nov 13. pii: 201707992. doi: 10.1073/pnas.1707992114. [Epub ahead of print] PMID: 29133411
GIV is Druggable
Earlier this year, the Garcia-Marcos laboratory reported detailed structural information describing how trimeric G proteins are activated by GBA motifs, protein segments capable of triggering G-protein signaling by a GPCR-independent mechanism. The work focused on GIV, a nucleotide exchange factor for Gαi3. Because we had previously shown that the GIV-Gαi3 interaction is required for cancer metastasis, we investigated if it could be disrupted by small molecules. The identification of such compounds would represent the first step in the development of novel anti-metastatic drugs, an urgently needed arm of current cancer therapeutic strategies.
Disrupting protein-protein interactions (PPIs) like the one established by GIV and Gαi3, however, is notoriously challenging. A significant hurdle to therapeutic development is demonstrating that a given PPI can be targeted by small molecules in the first place – i.e. they tend not to be “druggable.” To establish the druggability of our target, we combined computational approaches and wet laboratory techniques, drawing on insights gathered from our recent studies. We concluded disruption of the PPI target could indeed be achieved by small molecules and furthermore that the mode of action can be readily predicted by utilizing structural information.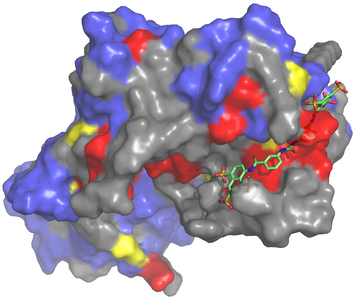
The work establishes a robust pipeline for the discovery and validation of inhibitors of the GIV-Gαi3 interface and identifies a small molecule that can serve in such a role. A limitation is that the small molecule we validated was not suitable for experimentation in cancer cells or patients. The study nonetheless provides an important proof of principle for the druggability of our target, success with which could be achieved by screening larger libraries of chemical compounds. Such high-throughput screens are currently underway in our laboratory.
This work involved collaboration with the group of Francisco J. Blanco, from the CIC-BioGUNE in Spain and was published in the journal Scientific Reports.
Reference
The Gαi-GIV binding interface is a druggable protein-protein interaction. DiGiacomo V, de Opakua AI, Papakonstantinou MP, Nguyen LT, Merino N, Blanco-Canosa JB, Blanco FJ, Garcia-Marcos M. Sci Rep. 2017 Aug 17;7(1):8575. doi: 10.1038/s41598-017-08829-7. PMID: 28819150
Faculty Appointed to NIH Study Sections
Dr. Konstanin Kandror was appointed to the National Institutes of Health, Cellular Aspects of Diabetes and Obesity (CADO) study section and Dr. Mikel Garcia-Marcos was appointed to the Molecular and Integrative Signal Transduction study section. These appointments are made based on "their demonstrated competence and achievement in their scientific discipline as evidenced by the quality of research accomplishments, publications in scientific journals, and other significant scientific activities, achievements and honors."
Mikel Garcia-Marcos Awarded Grunebaum Fellowship
Mikel Garcia-Marcos has been selected as the Karin Grunebaum Cancer Research Fellow for a second year in a row. The Grunebaum Faculty Research Fellowship is a BUSM annual faculty award that provides $25,000 in total funds to a selected faculty member for a period of one year. Several Faculty members of our Department, including Bob Varelas and Valentina Perissi, have been awarded this fellowship in the past. Dr. Garcia-Marcos plans to work on developing a novel therapeutic strategy against cancer metastasis that targets an unconventional mechanism of heterotrimeric G protein activation not mediated by surface receptors (GPCRs).