Leukemia and Lymphoma Laboratory
DENIS LABORATORY — CANCER RESEARCH CENTER
Welcome to the Denis Lab website.
The following is a summary of our research accomplishments and overall goals.
The lab is primarily interested in how gene expression controls cell growth in the immune system. Our central object of study is the ubiquitously expressed protein Brd2, which contains two mutually related “bromodomains,” and indirectly controls the transcription of target genes through chromatin modification and remodeling of nucleosomes. All of the work in our lab relates in one way or another to exploring the mechanisms of how this interesting and essential factor affects gene expression.
We have shown that in cancer, specifically aggressive leukemias and lymphomas, Brd2 levels are upregulated; artificially increasing Brd2 levels in B cells leads to their overproliferation and thence to aggressive and fatal B cell lymphoma in mouse models. Decreased Brd2 levels interfere with cell cycle progression and can cause immunodeficiency. This protein therefore functions like a kind of “thermostat” for proliferation, and given its fundamental nature, Brd2 likely has important biological roles far beyond the immune system.
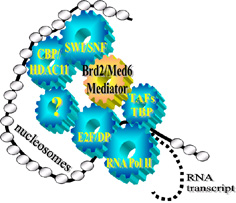 Brd2-anchored multiprotein complexes control cyclin A transcription through recruited E2F proteins and histone H4-directed HAT activity; as well as co-recruited transcription co-activators and co-repressors, and proteins of the SWI/SNF chromatin remodeling complex. Furthermore, chromatin immunoprecipitation establishes in synchronized cells that Brd2 is associated with the cyclin A promoter at both G1 and S phase of the cell cycle. Therefore, Brd2 likely functions as a scaffold that mediates access of transcriptional control proteins to chromatin. These observations imply that the Brd2 complex on chromatin has dynamic, time-ordered functions of transcriptional repression, then activation, then repression again as the cell progresses through S phase, when close control of cyclin A is essential for survival. A transcriptional model for cyclin A is shown at left.
Brd2-anchored multiprotein complexes control cyclin A transcription through recruited E2F proteins and histone H4-directed HAT activity; as well as co-recruited transcription co-activators and co-repressors, and proteins of the SWI/SNF chromatin remodeling complex. Furthermore, chromatin immunoprecipitation establishes in synchronized cells that Brd2 is associated with the cyclin A promoter at both G1 and S phase of the cell cycle. Therefore, Brd2 likely functions as a scaffold that mediates access of transcriptional control proteins to chromatin. These observations imply that the Brd2 complex on chromatin has dynamic, time-ordered functions of transcriptional repression, then activation, then repression again as the cell progresses through S phase, when close control of cyclin A is essential for survival. A transcriptional model for cyclin A is shown at left.
Brd2 is highly related to a Drosophila developmental factor called female sterile homeotic (fsh), and has homologs in yeast, zebrafish, frogs and other model organisms. The fsh gene is an upstream activator of the trithorax locus in Drosophila; fsh deficiency exacerbates the severity of trithorax mutations. This genetic regulatory system is important because the homologous human genes have been linked to lymphoid malignancy; mixed lineage leukemias in particular. Therefore, study of these and other molecular interactions in model systems, especially where chromatin effects can be measured, are likely to tell us a great deal about the origins of leukemia and lymphoma in humans, and ultimately, how to control and cure these diseases.