Substance Use Disorders
Substance use disorders (SUDs) are a major public health problem in the US. In 2013, 4.46 million Americans age 12 and older used cocaine in the past year.1 Cocaine dependence disproportionately affects African-Americans, who often experience greater legal consequences of cocaine use than European-Americans. The lifetime prevalence of opioid dependence is 0.3% and opioid use 1.1%.2 The estimated cost to society in 2002 for opioid abuse was $181 billion.3 Alcohol dependence is a common psychiatric disorder characterized by impaired control over alcohol consumption. Estimates of the lifetime and 12-month prevalence of the disorder in the U.S. population were 12.5% and 3.8%, respectively.4 The annual costs related to problem drinking in the United States were estimated to be $223.5 billion in 2006 due to health consequences, criminal behavior, and lost productivity.5 Cannabis remains the most widely used drug (besides nicotine) worldwide6 and in the US, where there has been a trend towards decriminalization based on a popular but erroneous7 perception that it is relatively harmless. Its use produces symptoms including craving,8 dependence,9 and drug seeking behavior,10 which are associated with misuse of other substances.
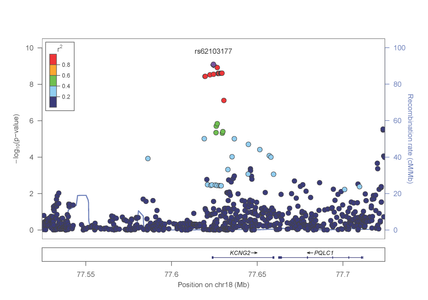
In the study focused on opioid dependence, published in Biological Psychiatry (http://www.sciencedirect.com/science/article/pii/S0006322313008263), genome-wide significant evidence of association was identified in the AA group with a SNP in the KCNG2 gene which encodes a neuronal voltage gated potassium channel (figure 1).
Figure 1: Association results from the KCNG2 gene on chromosome 18 with opioid dependence
SUDs have a strong genetic component. Family and twin studies have shown high heritability for dependence on nicotine, alcohol, cocaine, opiates and cannabis. The research team led by Dr. Lindsay Farrer and colleagues at Yale and the University of Pennsylvania have identified many gene loci for these SUDs by linkage,11-14 candidate gene,15-19 and genome-wide association study (GWAS)20-25 approaches.
However, these loci explain only a small proportion of the genetic variance. The “missing heritability” may be explained by other genetic mechanisms and gene*environment interactions. Examples of well-recognized exogenous factors affecting SUD risk include cultural inheritance from relatives, peers or societal practices, as well as traumatic life events. Endogenous risk factors that have been associated with SUD risk include personality and comorbid psychiatric disorders (PDs). We have examined the interaction of traumatic life events and co-morbid psychiatric illness with specific candidate genes in a hypothesis driven approach,26,27 and our findings are proof-of-principle supporting the idea that such interactions exist and that we are capable of identifying them. Our more recent studies which focused on the interaction of candidate genes with history of trauma,28 marital status,29 and post-traumatic stress disorder30 yielded promising findings.
Understanding the genetic variants and their interaction with other risk factors that predispose certain individuals to SUDs may lead to better diagnosis, treatment, and prevention approaches. In addition, differences in risk related to genetic make-up have important scientific and public policy implications.
Current Projects
Ongoing projects in the Biomedical Genetics division address a variety of research questions and use multiple approaches. We are conducting a whole exome sequence study to identify rare variants contributing to risk of opioid dependence. The contribution to SUD risk from interaction of genes with co-morbid psychiatric disorders, traumatic life events, early exposure to addictive substances, components of personality is being evaluated using innovative GWAS approaches.
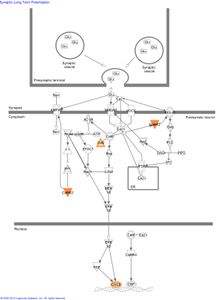
Several genes with the strongest evidence for association are clustered in biological pathways involved in the regulation of calcium and potassium levels and synaptic long term potentiation in neurons:
Figure 2: The role of genes identified in GWAS of AA subjects in the canonical pathway “synaptic long term potentiation.”
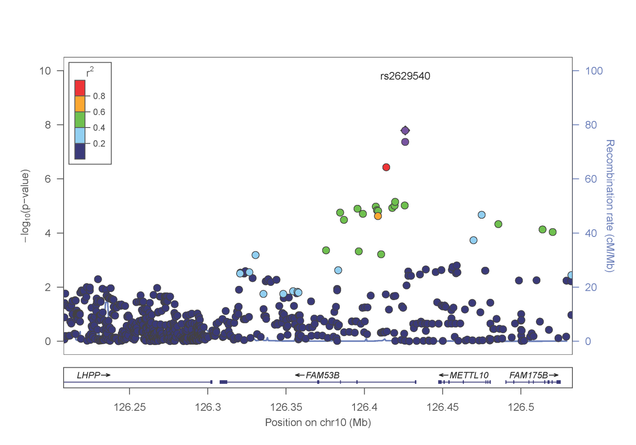
In a separate paper published in Molecular Psychiatry (http://www.nature.com/mp/journal/vaop/ncurrent/pdf/mp201399a.pdf), the investigative team reported a genome-wide significant association between risk for cocaine dependence and a SNP in FAM53B, a gene involved in regulating cell proliferation (figure 3). This finding was evident in both AA and EA samples. Intriguingly, several of the most significantly associated genes cluster in pathways related to calcium/potassium signaling.
Figure 3: Association results from the FAM53B gene on chromosome 10 with cocaine dependence.
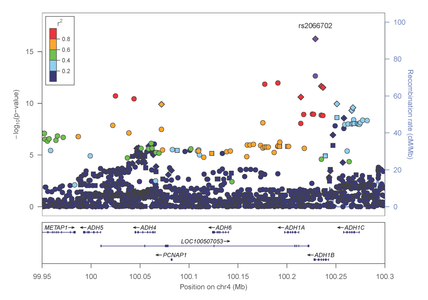
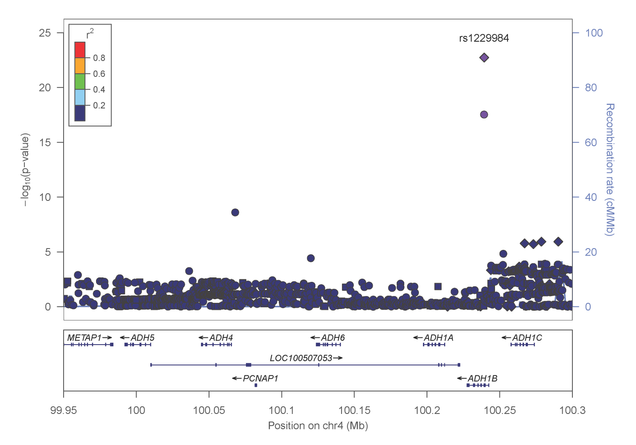
Most recently, Dr. Farrer, Dr. Sherva and their collaborators, identified association of alcohol dependence with several previously reported mutations in alcohol metabolizing enzymes. Importantly, the most significant findings were observed with two distinct non-synonymous (.i.e., predicted to be functionally relevant) mutations in the alcohol dehydrogenase 1B gene (ADH1B) that are population-specific (figures 4 and 5).
Figure 4: Association results within the alcohol metabolizing gene cluster on chromosome 4 in AAs
Figure 5: Association results within the alcohol metabolizing gene cluster on chromosome 4 in EAs
Faculty who conduct research in this area
References
- Substance Abuse and Mental Health Data Archive (SAMHDA). National Survey on Drug Use and Health, 2013 (ICPSR 35509) http://www.icpsr.umich.edu/icpsrweb/SAMHDA/studies/35509
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IVdrug abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007; 64:566-576.
- Office of National Drug Control Policy. The Economic Costs of Drug Abuse in the United States, 1992-2002. Washington, DC: Executive Office of the President (Publication No. 207303), 2004. www.ncjrs.gov/ondcppubs/publications/pdf/economic_costs.pdf
- Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum Genet. 2012; 131:779-789.
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 2011; 41:516-524.
- United nation office on drugs and crime (UNODC) world drug report 2012 (2012) 2–3.
- Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014; 371:879.
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci USA. 2009; 106:13016-13021.
- Weinstein AM, Gorelick DA. Pharmacological treatment of cannabis dependence. Curr Pharm Des. 2011; 17:1351-1358.
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008; 27:976-983.
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits Linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet Part B Neuropsychiatr Genet. 2005; 136B:45–52.
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, Kranzler HR. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006; 78:759–769.
- Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, Farrer L, Kranzler HR. Genomewide linkage scan for nicotine dependence: Identification of a chromosome 5 risk locus. Biol Psychiatry. 2007; 61:119–126.
- Panhuysen CI, Kranzler HR, Yu Y, Weiss RD, Brady K, Poling J, Farrer LA, Gelernter J. Confirmation and generalization of an alcohol dependence locus on chromosome 10q. Neuropsychopharm 2010; 35:1325-1332.
- Yu Y, Panhuysen C, Kranzler HR, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Weiss R, Brady K, Farrer LA, Gelernter J. Intronic variants in the dopa decarboxylase (DDC) gene are associated with smoking behavior in European-Americans and African-Americans. Hum Mol Genet 2006; 15:2192-2199.
- Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang B, Kranzler HR, Farrer LA. Haplotype spanning TTC12 and ANKK1, adjacent to the DRD2 locus, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet 2006; 15:3498-3507.
- Zhang H, Kranzler HR, Yang B-Z, Luo X, Gelernter J. The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol Psychiatry. 2008;13:531–543.
- Zhang H, Kranzler HR, Weiss RD, Luo X, Brady KT, Anton RF, Farrer LA, Gelernter J. Pro-opiomelanocortin gene variation related to alcohol or drug dependence: evidence and replications across family and population-based studies. Biol Psychiatry 2009; 66: 128–136.
- Sherva R, Kranzler HR, Yu Y, Poling J, Arias A, Anton R, Oslin D, Farrer LA, Gelernter J. Variation in nicotinic acetycholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology 2010; 35:1921-1931.
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Han S, Zhao H, Farrer LA. Genomewide association study of alcohol dependence: Significant findings in African- and European-Americans including numerous novel risk loci. Mol Psychiatry 2014; 19:41-49.
- Gelernter J, Sherva R, Koesterer R, Zhao H, Kranzler HR, Farrer LA. Genomewide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry 2014; 19:717-723.
- Gelernter J, Kranzler HR, Sherva R, Koesterer R, Sun J, Bi J, Almasy L, Zhao H, Farrer LA. Genomewide association study of opioid dependence and related traits: multiple associations mapped to calcium and potassium pathways. Biol Psychiatry 2014; 76:66-74.
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R, Zhao H, Farrer LA. Genomewide association study of nicotine dependence in American populations: identification of novel risk loci in both African- and European-Americans. Biol Psychiatry 2015; 77:493-503.
- Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, Zhao H, Farrer LA, Gelernter J. Genome-wide association study of maximum number of alcohol drinks in European Americans and African Americans. Alc Clin Exp Res 2015. In press.
- Sherva R, Wang Q, Kranzler HR, Zhao H, Koesterer R, Herman A, Farrer LA, Gelernter J. Genome wide association study of cannabis dependence severity reveals novel risk variants, genes previously implicated in schizophrenia risk, and shared risk with major depressive disorder. JAMA Psychiatry 2016. In press.
- Xie P, Kranzler HR, Zhang H, Oslin D, Anton R, Farrer LA, Gelernter J. Childhood adversity increases risk for nicotine dependence and interacts with alpha-5 nicotinic acetylcholine receptor genotype specifically in males. Neuropsychopharm 2012; 37:669-676.
- Xie P, Kranzler HR, Farrer L, Gelernter J. Serotonin transporter 5-HTTLPR genotype moderates the effects of childhood adversity on posttraumatic stress disorder risk: a replication study. Am J Med Genet B Neuropsychiatr Genet 2013; 159B:644-652.
- Xie P, Kranzler HR, Zhang H, Oslin D, Anton R, Farrer LA, Gelernter J. Childhood adversity increases risk for nicotine dependence and interacts with alpha-5 nicotinic acetylcholine receptor genotype specifically in males. Neuropsychopharmacology 2012; 37:669-676.
- Zayats T, Yang BZ, Xie P, Poling J, Oslin D, Farrer LA, Gelernter J. A Complex Interplay between Personality Domains, Marital Status and a Variant in CHRNA5 on the Risks of Cocaine, Nicotine Dependences and Cocaine-Induced Paranoia. PLos One 2113; 2013; 8:e49368
- Li D, Zhao H, Kranzler HR, Oslin D, Anton RF, Farrer LA, Gelernter J. Association of COL25A1 with co-morbid antisocial personality disorder (ASPD) and substance dependence. Biol Psychiatry 2012; 71:733-740.