Sam Thiagalingam, Ph.D.

Sam Thiagalingam, Ph.D.
Associate Professor of Medicine
Department of Medicine, Biomedical Genetics Section
Pathology & Laboratory Medicine
Post-Doctoral Fellow., Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
Ph.D., The Johns Hopkins University
M.S., Bowling Green State University
B.Sc (Hons.)., University of Jaffna
Awards and Honors
2019 – Carter Award for Diversity and Cancer Equity, BU-BUMC Cancer Center
2008 – Susan G. Komen for the Cure Investigator Initiated Research Award
2006 – NARSAD/Brain & Behavior Research Foundation NARSAD Independent Investigator Award
2006/2007 – NARSAD Dr. Walter F. Nichols Investigator
2001 – USAMRMC Career Development Award, Department of Defense BCRP
2000 – Smith Family Foundation/The Medical Foundation New Investigator Award (Dolphin Trust Investigator)
Graduate Program Affiliations
Genetics & Genomics
Molecular & Translational Medicine
Pathology & Laboratory Medicine
Center Affiliations
Boston University-Boston University Medical Center Cancer Center
Genome Science Institute (GSI)
Research Interests
MMMN cancer progression models for breast and colon cancers, cancer stem cells, epigenetic memory, epigenetic regulation of cancer metastasis and major psychiatric disorders and personalized therapy.
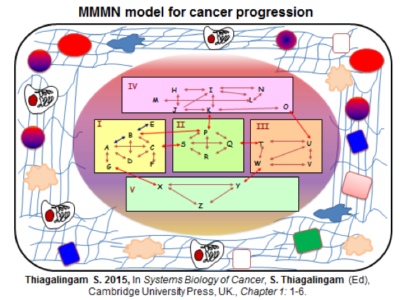
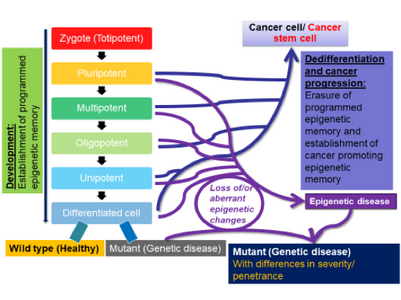
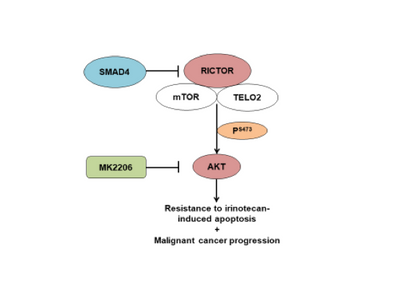
Dr. Thiagalingam’s primary research interest in cancer genomics and biology has been to elucidate multi-modular molecular network (MMMN) cancer progression models as the road map to dissect the complexity inherent to cancer and to design therapeutic strategies. As a post-doctoral fellow, he showed that loss of heterozygosity (LOH) at 18q targeted the SMAD4 gene inactivation in colon cancer. As a result, the multi-step colon cancer progression model (i.e., “Vogelgram”) was revised to include SMAD4. Subsequently, he was involved in the identification of a family of five novel SMAD genes. The discovery of SMAD genes provided the missing link between the TGFβ receptors and the downstream signaling end effects. As a faculty member at Boston University School of Medicine, he continued to study the SMAD genes with the goal of deciphering their role(s) in cancer progression. Dr. Thiagalingam’s laboratory showed that loss of Smad4 promotes the metastatic potential of colon cancer cells due to loss of metastasis suppressor function leading to the enhanced expression of effectors such as VEGF, MMP9, and GLUT1 with corresponding increases in cell migration, aerobic glycolysis and resistance to 5’-fluoruracil-mediated apoptosis. Recently, with the aid of proteomics approach, Thiagalingam lab uncovereded hyperactivation of the mTORC2 pathway in SMAD4 defective colon cancer, and found that it could be a target for precision cancer therapy to enhance sensitivity to chemotherapy resistant colon cancer.
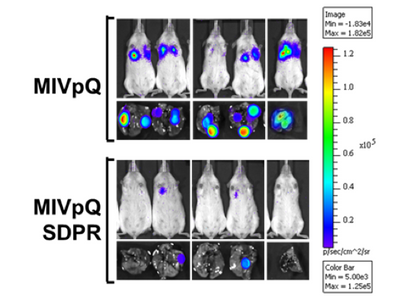
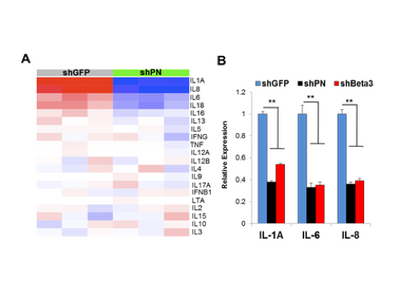
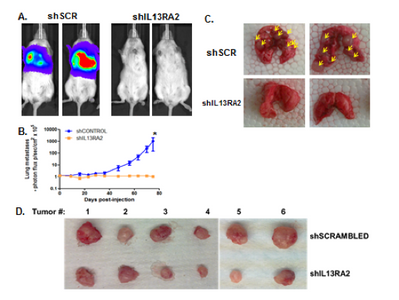
Dr. Thiagalingam’s laboratory has also made several contributions to the understanding of breast cancer progression and metastasis. Continuing with the interest in the role of TGFβ-Smad signaling pathway in breast cancer, initial studies uncovered that SMAD8 is a potential diagnostic and/or prognostic biomarker and a target for epigenetic silencing via DNA hypermethylation. In a recent proof of principle study using a breast cancer progression model system, his group discovered that overactive TGFβ signaling events are responsible for sustaining an altered cancer epigenome (i.e. “epigenetic memory”) defined by aberrant DNA methylation patterns affecting a subset of critical target genes involved in breast cancer. Since metastatic disease represents a significant clinical problem as it is incurable and remains as the major cause of death in the majority of breast cancer patients, Thiagalingam lab decided to identify novel metastasis suppressor and promoter genes that may have eluded the previous research efforts. To achieve this goal, they generated gene expression profiles of a breast cancer model system consisting of cell lines of the same genetic lineage representing the normal, benign, in situ carcinoma and the metastatic stages. By examining the genes that are overexpressed (i.e., candidate metastasis promoter genes) or lost in expression (i.e., candidate metastasis suppressor genes) in a metastasis specific manner in the model system, using meta-analyses of the pre-existing data on gene expression patterns of the tumor samples, and by evaluating for significant correlation to relapse of breast cancer in patients who underwent therapy, the Thiagalingam lab identified a metastasis promoter gene, IL13Rα2, and a candidate metastasis suppressor gene, of the serum deprivation response (SDPR). While IL13Rα2 has been previously implicated as a metastasis promoter gene in other cancers, the role of SDPR was uncovered for the first time as a candidate metastasis suppressor gene through these efforts. A related research interest for the Thiagalingam lab has been to study the cancer stem cells (CSCs) regarded as the therapeutically resistant subpopulation of metastatic cancer cells responsible for disease recurrence. On this front, Thiagalingam lab found that periostin (POSTN), an extracellular matrix molecule secreted by the CSCs themselves is an essential constituent of the metastatic tumor microenvironment and plays a role in their maintenance by regulating critical cytokines.
Overall, ultimate goal of the Thiagalingam lab is to contribute towards the development of a MMMN cancer progression models for breast and colon cancers and help to uncover novel diagnostic/prognostic biomarkers and targets for therapy as well as developing effective therapeutic strategies and agents for the metastatic cancer.
In addition to studying the various aspects of cancer, Dr. Thiagalingam has also been interested in taking an interdisciplinary approach to studying other complex diseases such as schizophrenia (SCZ) and bipolar disorder (BD). Since the genetic make-up, along with functional genetic polymorphisms are insufficient by themselves to provide a molecular basis for the pathogenesis of the majority of individuals with major psychiatric disorders such as SCZ and BD, Dr. Thiagalingam teamed up with Dr. Abdolmaleky, a physician scientist, to study the epigenetic basis of these major psychiatric disorders. Pioneering studies performed in Dr. Thiagalingam’s laboratory provided the first conclusive evidence for DNA hypermethylation silencing of reelin (RELN) and hypomethylation associated overexpression of the membrane-bound catechol-O-methyltransferase (MB-COMT) in SCZ and BD. Recent gene expression profiling studies by Drs. Thiagalingam and Abdolmaleky of left and right post-mortem brain samples from controls and patients with SCZ found support for asymmetric expression of many genes involved in the TGFB super family signaling pathways and those known to establish left-right asymmetry. These research efforts are continuing with the goal of unravelling correlations between the genomic and epigenomic alterations in schizophrenia and bipolar disorder to pathogenesis to identify effective therapeutic targets and agents.
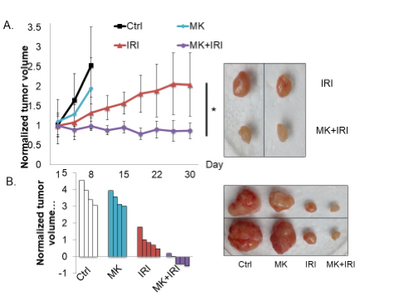
SMAD4-/- xenografts.

Labratory Plasmids: https://www.addgene.org/Sam_Thiagalingam/
Photos from the Lab:























Selected Publications:
- Abdolmaleky HM, Zhou JR, Thiagalingam S. 2021. Cataloging recent advances in epigenetic alterations in major mental disorders and autism. Epigenomics. 2021 Aug;13(15):1231-1245. doi: 10.2217/epi-2021-0074. Epub 2021 Jul 28. PMID: 34318684. View in: PubMed
- Thiagalingam S. 2020. Epigenetic memory in development and disease: unraveling the mechanism. Biochim Biophys Acta Rev Cancer. 2020 Jan 23; 1873(2):188349. View in: PubMed
- Wong CK, Lambert AW, Ozturk S, Papageorgis P, Lopez D, Shen N, Sen Z, Abdolmaleky HM, Győrffy B, Hui F, and Thiagalingam S. 2020. Targeting RICTOR sensitizes SMAD4-negative colon cancer to Irinotecan. Mol Cancer Res. 2020 Mar; 18(3):414-423. View in: PubMed https://www.drugtargetreview.com/news/54770/rictor-mtorc2-pathway-inhibition-could-make-chemotherapy-more-effective/; https://medicalxpress.com/news/2020-01-molecular-therapeutic-colon-cancer.html
- Wong CK, Gromisch C, Ozturk S, Papageorgis P, Abdolmaleky HM, Reinhard BM, Thiagalingam A, Thiagalingam S. 2019.MicroRNA-4417 is a tumor suppressor and prognostic biomarker for triple-negative breast cancer. Cancer Biol Ther. 2019; 20(8):1113-1120. View in: PubMed
- Abdolmaleky HM, Gower A, Wong CK, Cox JW, Thiagalingam A, Zhang X, Shafa R, Sivaraman V, Zhou J-R, and Thiagalingam S. 2018. Aberrant transcriptomes and DNA methylomes define pathways that drive pathogenesis and loss of brain asymmetry in schizophrenia and bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2019 03; 180(2):138-149. View in: PubMed
- Ozturk S, Papageorgis P, Wong CK, Lambert AW, Abdolmaleky HM, Thiagalingam A, Cohen HT, Thiagalingam S. SDPR functions as a metastasis suppressor in breast cancer by promoting apoptosis. Proc Natl Acad Sci U S A. 2016; 113(3):638-43. View in: PubMed Editors’ choice: Science: Vol. 351, Issue 6271, pp. 351. http://science.sciencemag.org/content/351/6271/twil; In the news: http://health-innovations.org/2016/01/11/major-epigenetic-metastasis-supressor-identified-for-breast-cancer/
- Lambert AW, Wong CK, Ozturk S, Papageorgis P, Raghunathan R, Alekseyev Y, Gower AC, Reinhard BM, Abdolmaleky HM, Thiagalingam S. Tumor Cell-Derived Periostin Regulates Cytokines That Maintain Breast Cancer Stem Cells. Mol Cancer Res. 2016; 14(1):103-13. View in: PubMed Highlights: http://mcr.aacrjournals.org/content/14/1/1.full.pdf+html; In the news: http://bit.ly/1WOWyD5
- Papageorgis P, Ozturk S, Lambert AW, Neophytou CM, Tzatsos A, Wong CK, Thiagalingam S., Constantinou AI. Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress breast cancer lung metastasis. Breast Cancer Res. 2015; 17(1):98. View in: PubMed In the news: http://tinyurl.com/o2c9net; http://tinyurl.com/pflox4o
- Thiagalingam S. Systems Biology of Cancer, S. Thiagalingam (Ed), Cambridge University Press, UK, 2015; Chapters 1-32: 1-531. www.cambridge.org/9780521493390
- Abdolmaleky HM, Zhou JR, Thiagalingam S. An update on the epigenetics of psychotic diseases and autism. Epigenomics. 2015; 7(3):427-49. View in: PubMed
- Papageorgis P, Cheng K, Ozturk S, Gong Y, Lambert AW, Abdolmaleky HM, Zhou JR, Thiagalingam S. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011; 71(3):998-1008. View in: PubMed
- Papageorgis P, Lambert AW, Ozturk S, Gao F, Pan H, Manne U, Alekseyev YO, Thiagalingam A, Abdolmaleky HM, Lenburg M, Thiagalingam S. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 2010; 70(3):968-78. View in: PubMed
- Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, Gao F, Smith CL, Shafa R, Aeali B, Carnevale J, Pan H, Papageorgis P, Ponte JF, Sivaraman V, Tsuang MT, Thiagalingam S. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006; 15(21):3132-45. View in: PubMed
- Thiagalingam S. A cascade of modules of a network defines cancer progression. Cancer Res. 2006; 66(15):7379-85. View in: PubMed
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S., Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005; 134B(1):60-6. View in: PubMed
- Thiagalingam S, Laken S, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B, Lengauer C. Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc Natl Acad Sci U S A. 2001; 98(5):2698-702. View in: PubMed
- Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996; 13(3):343-6. View in: PubMed
- Riggins GJ, Thiagalingam S, Rozenblum E, Weinstein CL, Kern SE, Hamilton SR, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mad-related genes in the human. Nat Genet. 1996; 13(3):347-9. View in: PubMed
- Thiagalingam S, Grossman L. The multiple roles for ATP in the Escherichia coli UvrABC endonuclease-catalyzed incision reaction. J Biol Chem. 1993; 268(24):18382-9. View in: PubMed
- Grossman L, Thiagalingam S. Nucleotide excision repair, a tracking mechanism in search of damage. J Biol Chem. 1993; 268(23):16871-4. View in: PubMed
- Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993; 362(6423):857-60. View in: PubMed
- Kern SE, Pietenpol JA, Thiagalingam S, Seymour A, Kinzler KW, Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992; 256(5058):827-30. View in: PubMed