Roussarie Lab
Our Research:
Selective neuronal vulnerability: leveraging this underexplored property of Alzheimer’s, to molecularly dissect the disease
Like almost all neurodegenerative diseases, Alzheimer’s disease in its earliest stages only affects very specific sets of neurons. In the majority of patients, neurons from the layer II of entorhinal cortex (ECII) are the first ones to accumulate pathological lesions and die. These neurons are crucial for assembling higher-order sensory information into new memories.
The vulnerability of ECII neurons is very poorly understood. We think that understanding what differentiates vulnerable neurons from more resistant neurons might open new therapeutic avenues, a necessity for the Alzheimer’s field. Vulnerability is a complex feature and likely results from a number of genes enriched/impoverished in ECII neurons, in addition to the particular neuronal and glial environment surrounding these neurons.
Using cell-type specific profiling techniques, and systems-level functional genomics, we demonstrated that ECII neurons possessed intrinsic features that could make them more vulnerable (Roussarie, Yao et al. Neuron, 2020). Further searching for hub genes driving pathological processes in vulnerable neurons, we identified a proto-oncogene, DEK, which regulates both tau accumulation and neuronal excitability (Rodriguez-Rodriguez et al., bioRxiv, 2022). We now want to continue exploring vulnerability to uncover the earliest events leading to neurodegeneration.
Some of our major projects include:
– Map cellular diversity within the EC across species
– Develop an in vitro model for vulnerable neurons
– Identify more drivers of neuronal vulnerability (which could become therapeutic targets) to gain insight into the different processes that regulate early stages of AD
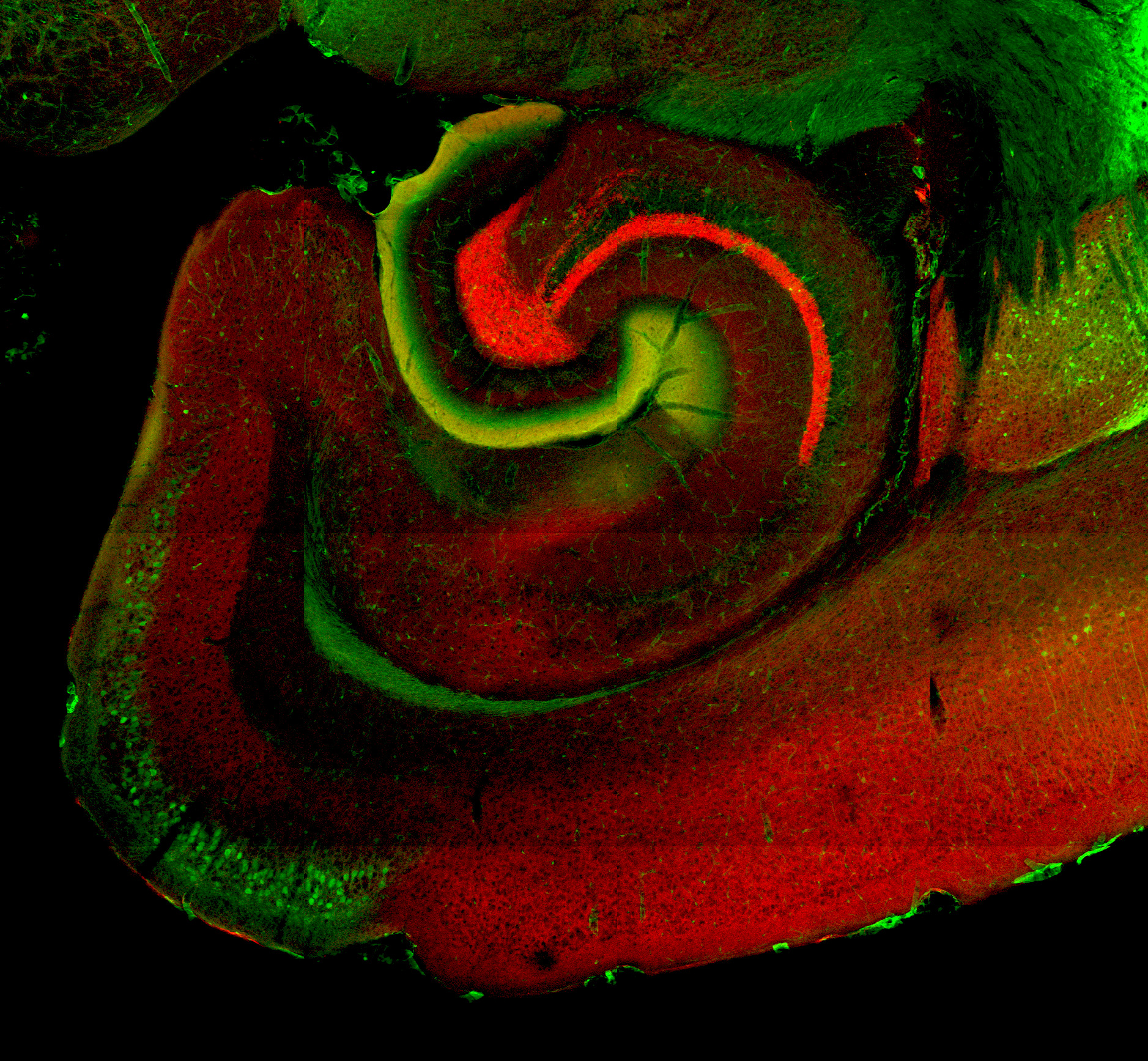
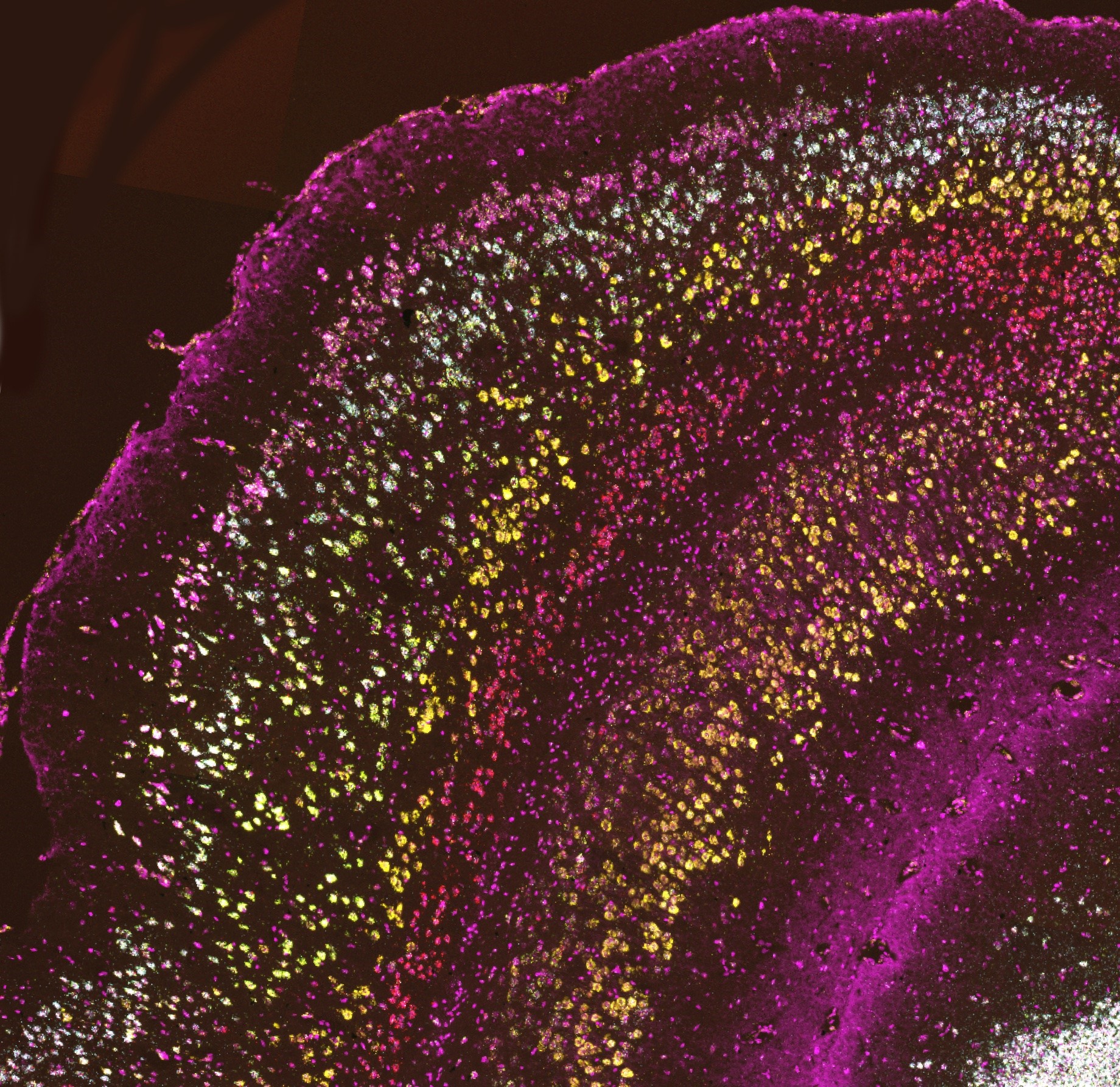
Map cellular diversity within the EC across species.
Using different technologies (multiplexed in situ hybridization, single-nucleus RNAseq, in situ sequencing) and different species (mouse, monkey, human), we want to identify subtypes of ECII neurons, beyond the traditional distinction between lateral and medial EC.
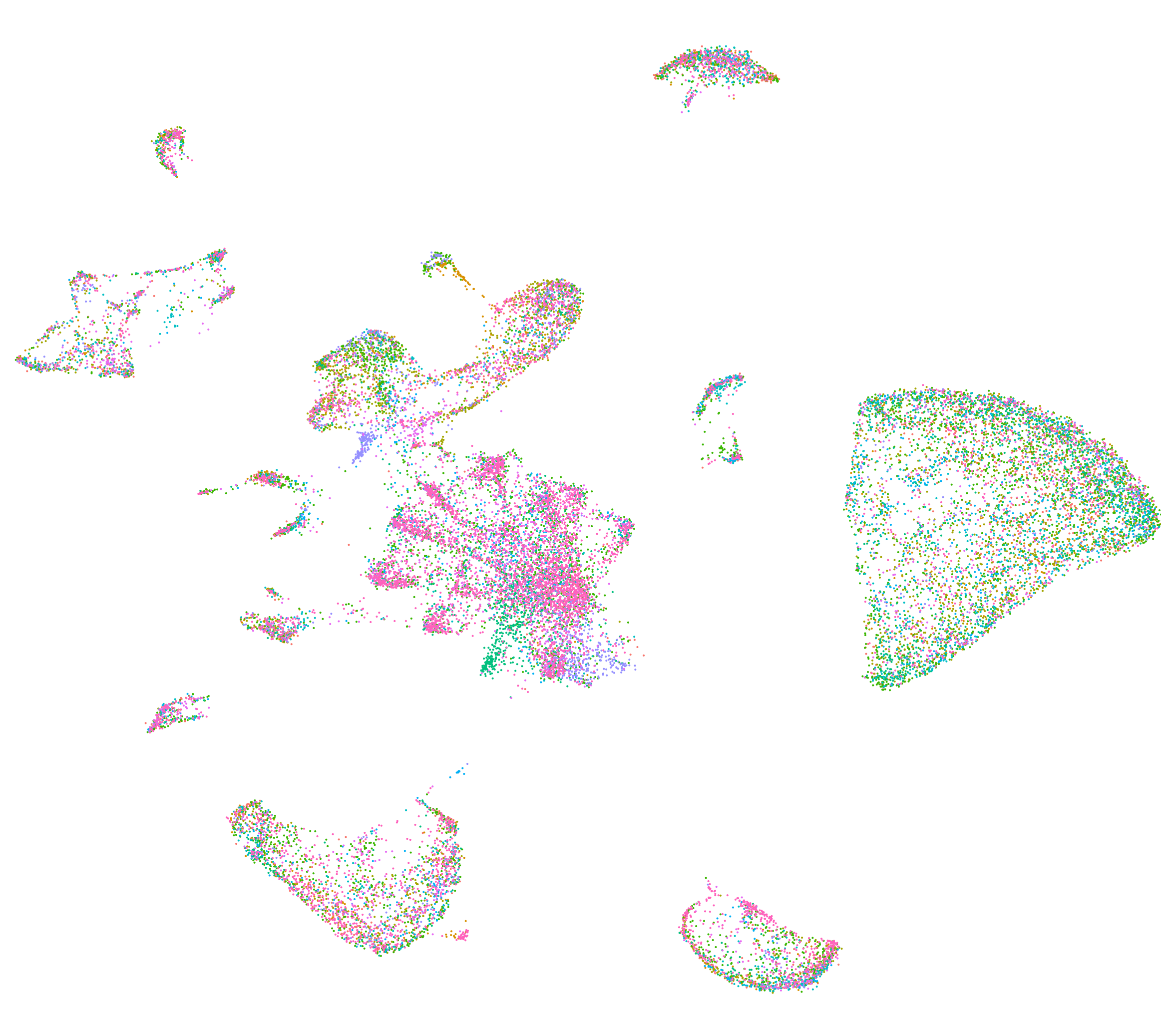
t-SNE plot of excitatory neurons in the mouse EC
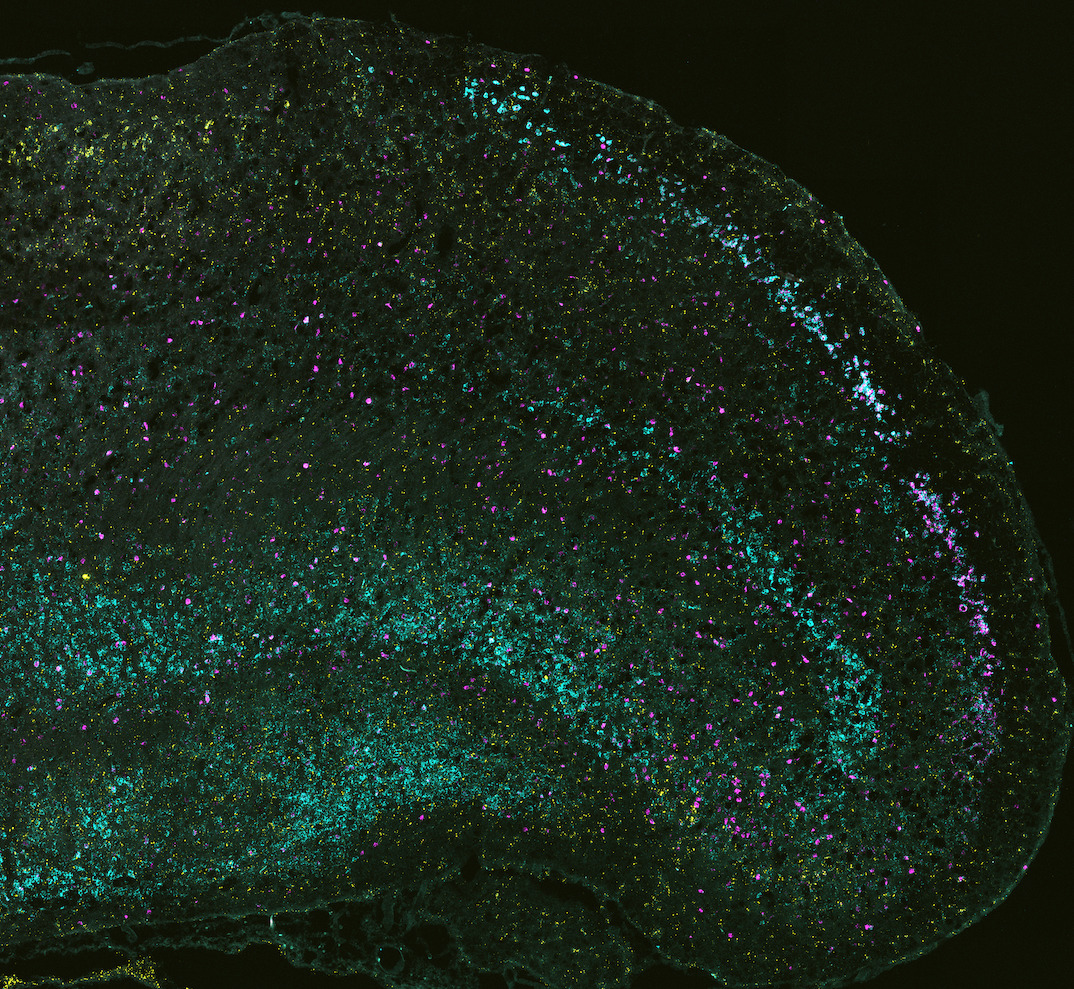
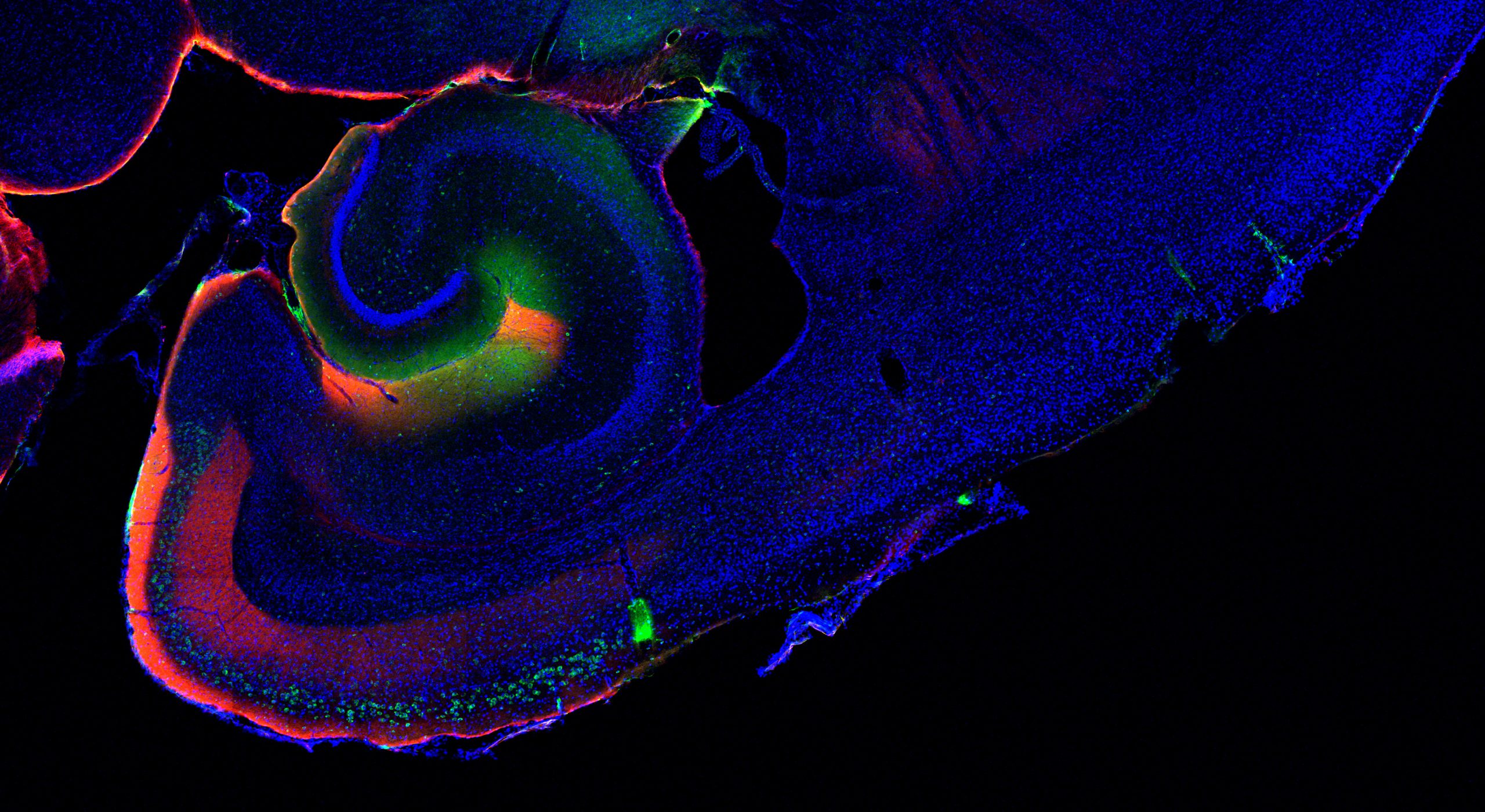
In situ hybridization for three markers for different subtypes of lateral ECII neurons
We want to test the molecular profiles, the connectivity patterns, and synaptic properties of these subtypes.
We also want to compare these subtypes across species.
Neurofibrillary tangle pathology does not start uniformly in this very heterogeneous region. It is thus indispensable to understand these neuron populations in their full complexity.
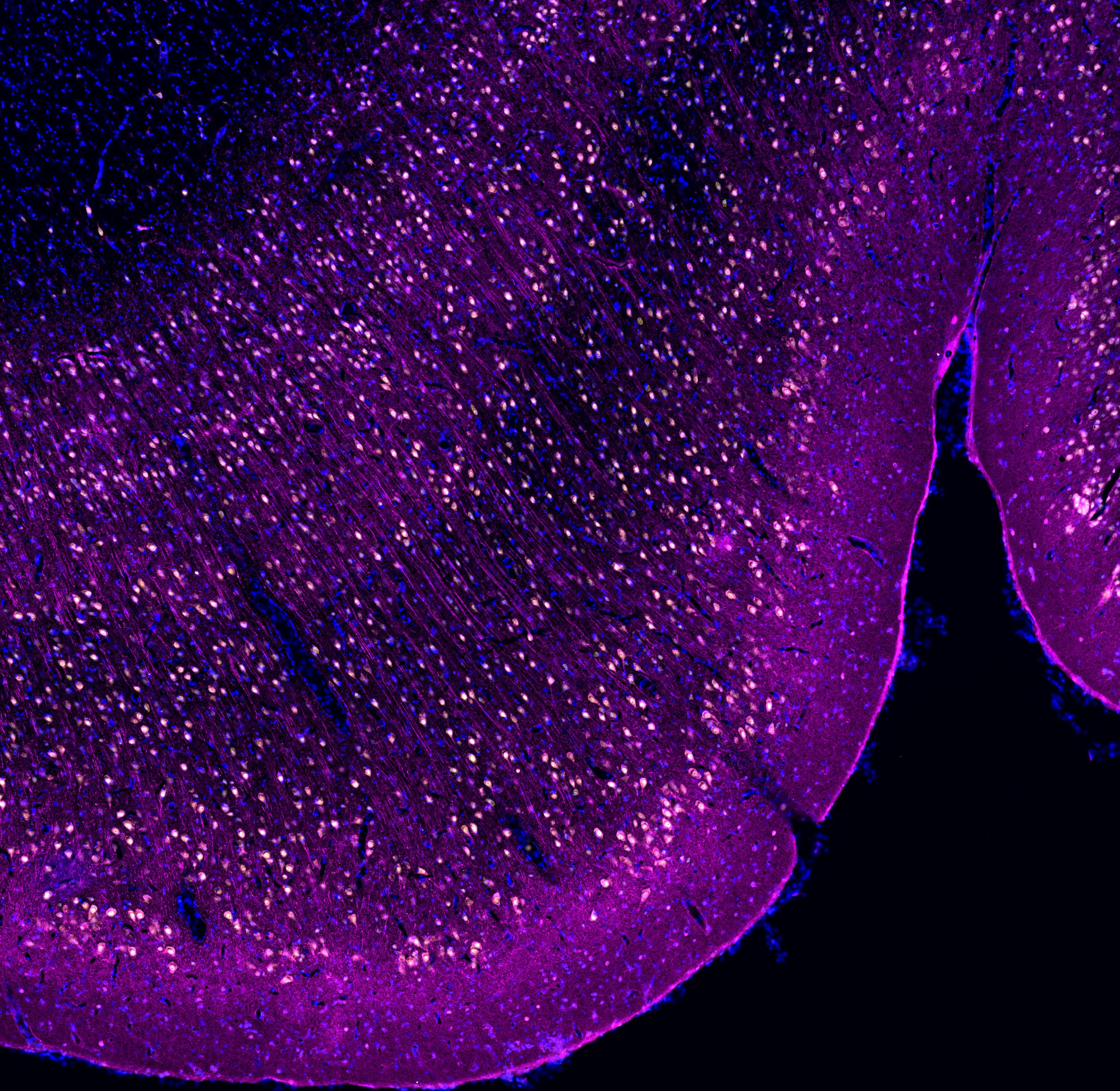
Detection of ECII neurons in the macaque EC
Back
Identify early drivers of neuronal pathology.
Our goal is to identify genes, that very early on during the disease process in vulnerable neurons, are responsible to pathological changes culminating in neurodegeneration.
We are thus looking for genes that are both:
– dysregulated transcriptionally at early presymptomatic stages of the disease. For this we use single-nucleus RNAseq and spatial transcriptomics on postmortem human EC.
– predicted to be functionally associated with tau pathology as per our functional network analysis (Roussarie et al., Neuron, 2020)
We recently identified two genes, the splice factor PTBP1 and the proto-oncogene DEK that can regulate tau in ECII neurons (respectively the splicing of tau exon 10, and the total levels of tau). We are currently investigating if these genes are dysregulated in early stages of the disease.
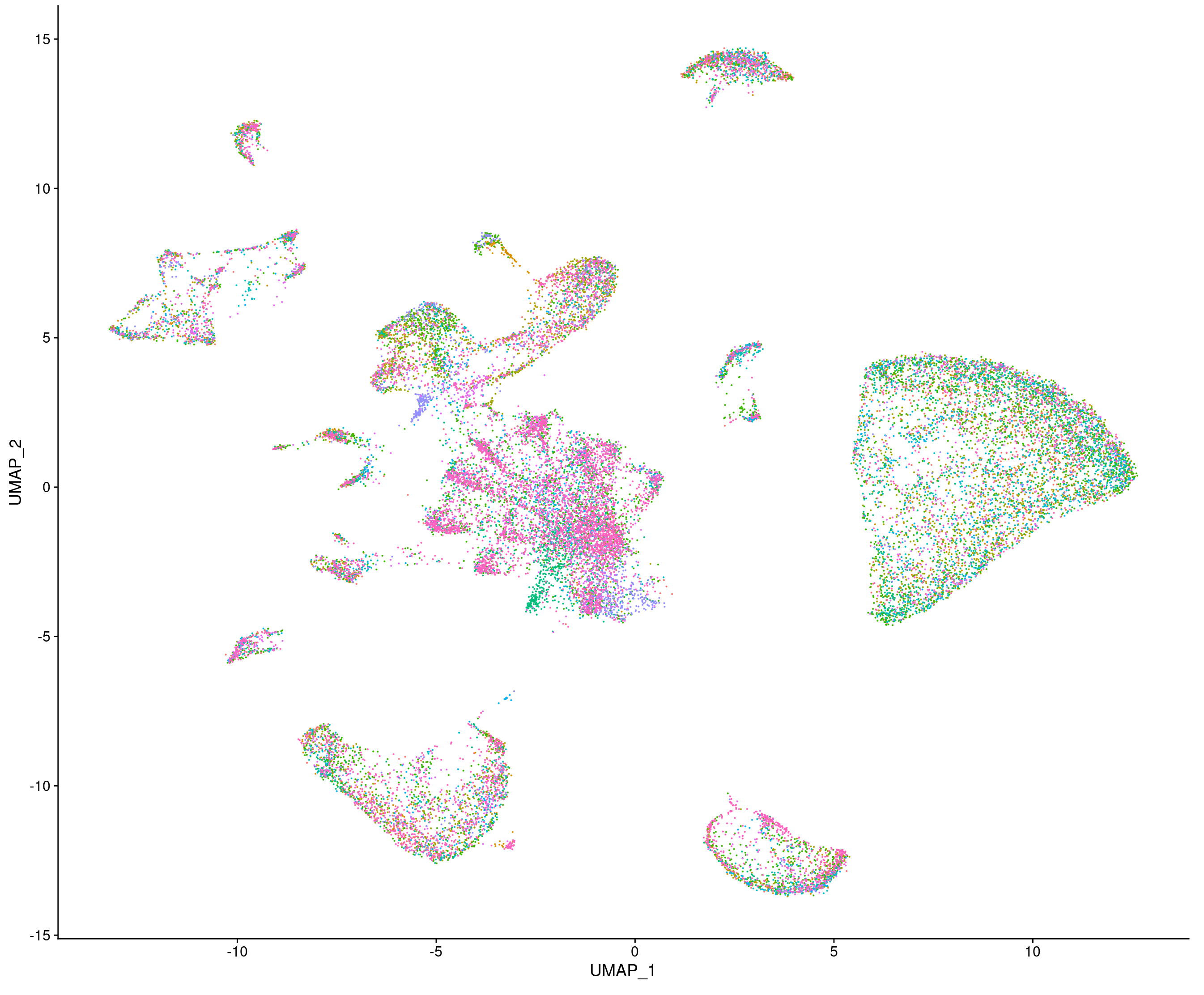
UMAP dimensionality reduction plot for single-nucleus RNAseq samples from control and presymptomatic AD EC.
We are modulating the top gene candidates in the EC of AD mouse models, or in our EC in vitro systems to validate that they are indeed driving tau pathology.
We are modulating the top gene candidates in the EC of AD mouse models, or in our EC in vitro systems to validate that they are indeed driving tau pathology.
Back
Develop an in vitro model for vulnerable neurons.
Research on selective neuronal vulnerability in Alzheimer’s is slowed down by the absence of good in vitro models for these very vulnerable neurons.
We optimized the culture of primary EC neurons from mouse embryos and used these cultures for testing vulnerability gene candidates.
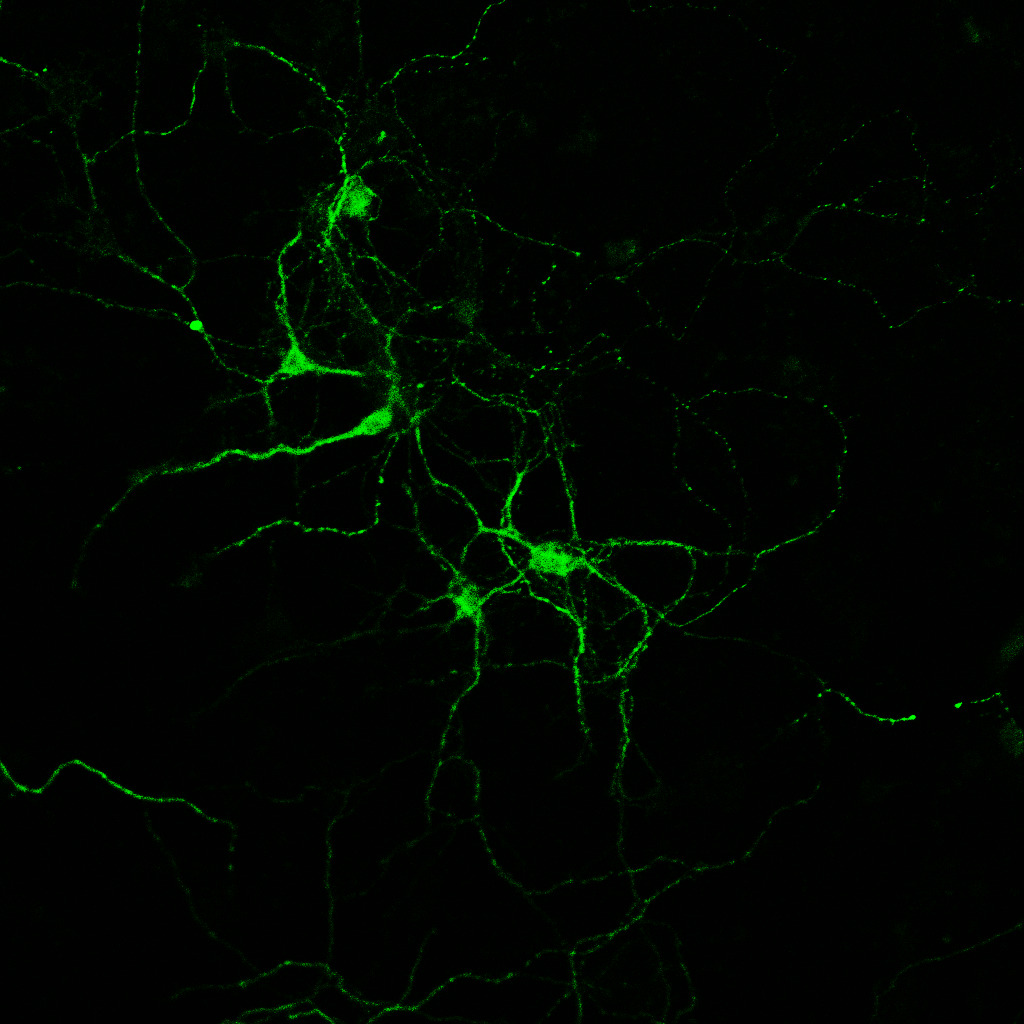
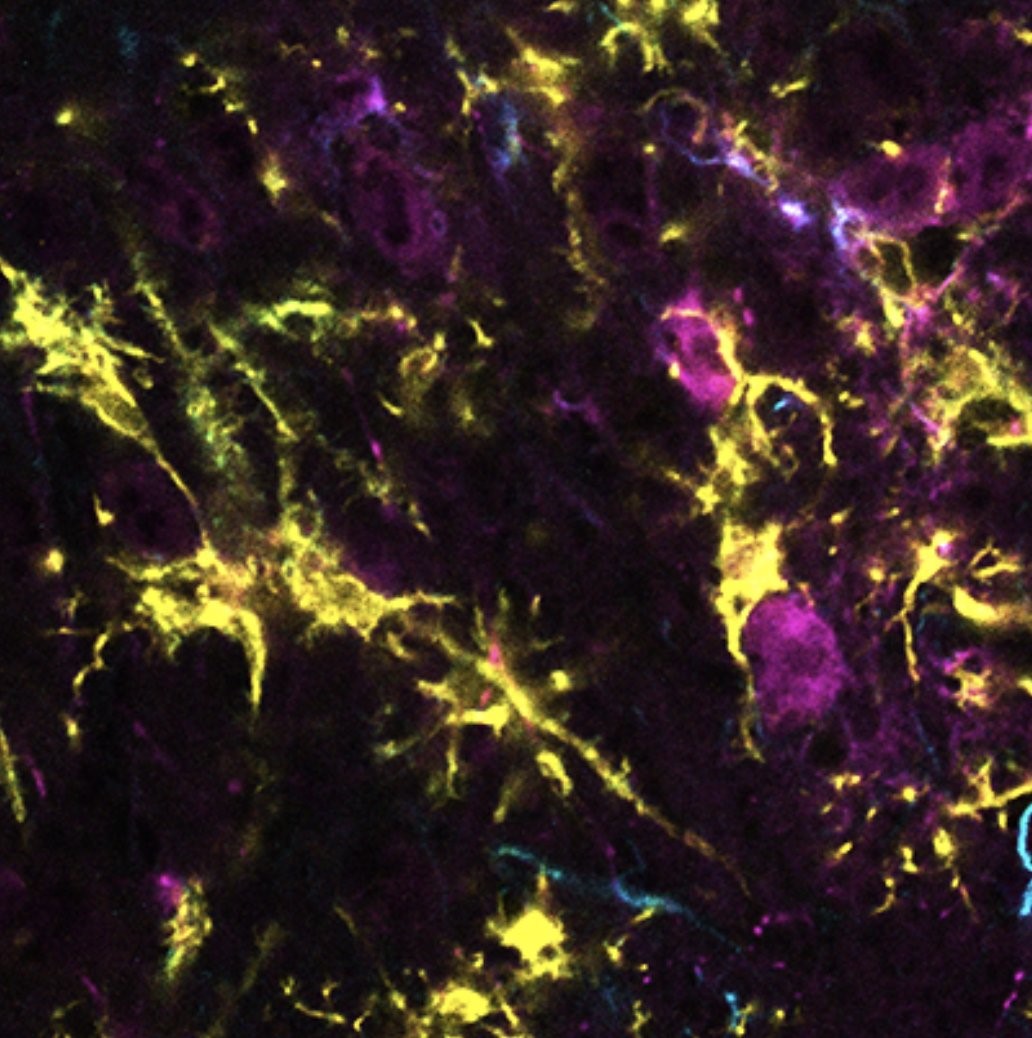
mouse primary neuron culture from EC after silencing of a candidate vulnerability gene. Phosphorylated tau is labeled in green.
In collaboration with Dr. Yao (Rice University), we are also searching for transcription factors that could drive ECII identity, using ECII-specific molecular profiles at different times during ECII development, as well human transcriptomic data.
We are using candidate transcription factors in induced pluripotent stem cells, and in transdifferentiating fibroblasts to make ECII-like neurons.
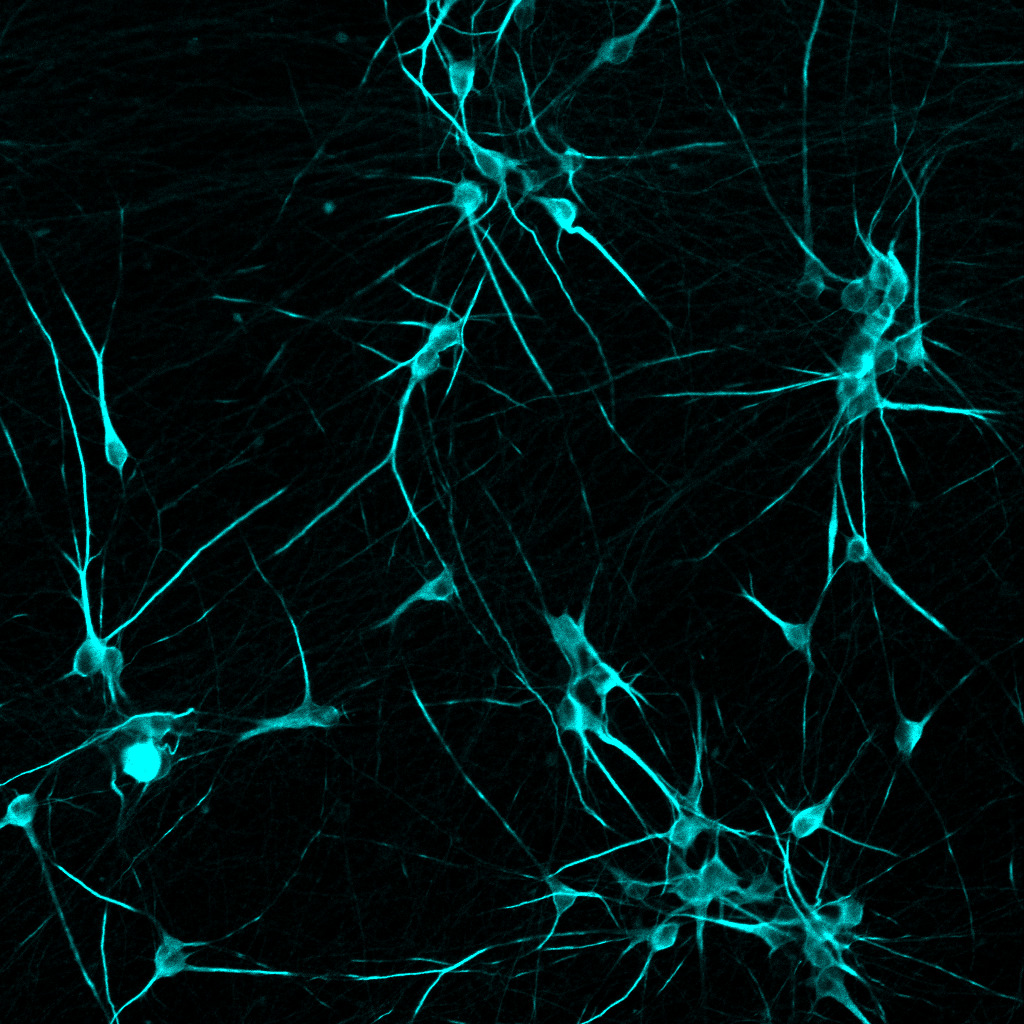
human iPSC-derived neurons labeled with MAP2 (cyan).
Back

Lab Members
As of 6/10/22
Manas Dhanuka
Masters Student
Irena Feng
MD-PhD Student
Saiyan Joseph
BU Undergraduate Student
Myles Joyce
Masters Student
Han Kahvecioglu
BU Undergraduate Student
Ana Morello Megias
PhD Student
Muriel Ruppert
STaRS Student
Emily Yang
Lab Manager
Patricia Rodriguez-Rodriguez PhD
Key Collaborator
Assistant Professor – Karolinska Institute
Roussarie Lab News
From Twitter
Resources
Contact Us
Roussarie Lab
72 East Concord Street, L817
Boston, MA 02115
Phone: 617-358-0811
Email: jproussa@bu.edu
Alumni
Postdocs:
Patricia Rodriguez-Rodriguez 2018-2021
Research Assistants:
Isabella Salas-Allende 2019-2021
Zakary Plautz 2017-2019
Mallory Brown 2015-2017
Assia Mouri 2014-2015
Quigly Dragotakes 2014-2016
Christian Albornoz 2011-2013
Shirin Kasturia 2010-2011
Undergraduate student:
Sophia Andreadis 2016, 2019
Funding Sources