Alzheimer’s Disease
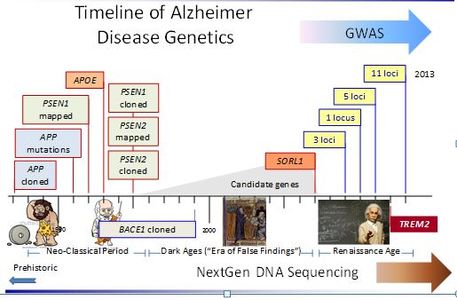
While great strides have been made in the genetics and biology of Alzheimer disease (AD), mechanisms leading to disease are not fully understood. Advanced age, a positive family history of dementia, and the ɛ4 allele of apolipoprotein E (APOE) are three prominent risk factors. Our intensive studies of these factors and genome-wide association studies have resulted in a better understanding of the genetic basis and age dependent penetrance of the disorder, as well as provided several clues about the interaction of genetic and non-genetic factors on disease risk. Our work has provided several lines of evidence supporting the idea that risk factors for vascular disease increase AD susceptibility and/or influence the expression of the disorder. We also identified that intracellular protein trafficking is an important pathway leading to AD.
AD Researchers in the Biomedical Genetics section direct and collaborate in several projects aimed at identifying novel genetic factors, and examining interactions between genes, non-genetic risk factors, and novel biomarkers for developing AD. We also co-direct efforts for several very large consortia including the Alzheimer Disease Genetics Consortium (ADGC), Alzheimer Disease Sequencing Project, and International Genomic Alzheimer Project (IGAP). Results of these studies will speed the development of new diagnostic techniques and treatments for AD.