Laboratory for Cortical Organization & Architectures
Our Research
We aim to identify and compare key morphological features of neurons, axons, and neuropil in diverse cortical areas, and to understand how these 1) enable area-specific functionality and 2) may be associated with differential vulnerability.Ongoing collaborations support investigations of macaque monkey (Dr. Alvaro Duque, Yale University) and the hippocampal-entorhinal network in humans (Dr. Jean Augustinack, MGH). In a recently completed collaboration (with Dr. Rick Born, Harvard Medical School), we tested several techniques, including x-ray nano- holography, for probing the microcircuitry of feedback cortical architectures in the macaque monkey.
The general lab program is a continuation from decades of research on single axon topologies at the light microscopic level. A new direction, revisiting previous interests (e.g., Rockland and DeFelipe (2012)), is fine scale analysis of white matter organization in monkey and human. As a unique resource, we refer to several brains processed in uninterrupted serial sections after injections of the anterograde tracer biotinylated dextran amine (BDA). Click on boxes below for more.
Underway are research conversations relating to neuroanatomy-tractography with several colleagues in the newly formed Society for International Tractography.
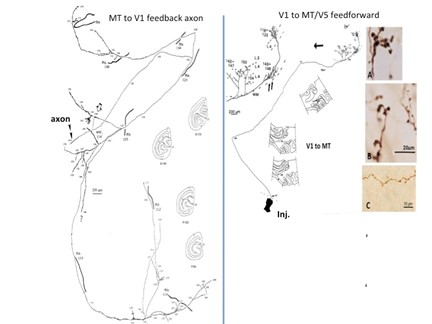
from Rockland and Knutson, 2000 / from Rockland, 1995
Feedback Cortical Connections
Collaborators

Prof. Rick Born (Harvard Medical School)

Dr, Vladimir Berezovskii (Harvard Medical School)
Click here for more on the collaborators.
Background.Feedback cortical connections were identified in the 1970’s on the basis of a distinct laminar phenotype; namely, these originate from excitatory pyramidal neurons in layers 2, 3A, and 6 and terminate mainly in layer 1, sometimes with collaterals in the deeper layers. Feedback connections are one of several distinguishable output cortical fiber systems (with callosal, cortico-thalamic and other cortico-subcortical connections), but the sharp laminar contrast with “feedforward” cortical connections has attracted the most interest. Although the feedforward-feedback distinction applies best to the early sensory cortical pathways, the dichotomy is often generalized to any two-way pathway; for example, connections between prefrontal and parietal areas. In this broader guise, feedback connections have been an important inspiration for ideas of hierarchical organization (e.g., Felleman and Van Essen, 1991) and, more recently, predictive coding (S. Shipp, 2016 https://pubmed.ncbi.nlm.nih.gov/27917138/ ).
Much of my research career has been involved with visual cortical feedback connections, starting with my Ph.D. thesis (Rockland and Pandya, 1979), and continuing with several investigations of the distinctive topological features of feedback and feedforward connections at the level of single axons (Rockland and Virga, 1989; Rockland and Knutson, 2000, among others (reviewed in Rockland, 2020 https://pubmed.ncbi.nlm.nih.gov/31925518/ )).
Astonishingly, however, the anatomical features of feedback connections are only partly known. Subtypes of neurons (at the transcriptomic level)? Postsynaptic dendritic targets in layer 1? Convergence with other inputs (from the thalamus, basolateral amygdala, and others) to layer 1? Area specializations across the early visual pathway (V1, V2, V4, MT)?
To continue addressing these issues, I have recently joined with Rick Born and his colleagues, who have also had a long-term interest in feedback, but mainly using a physiological approach (Gomez-Laberge et al., 2016 https://pubmed.ncbi.nlm.nih.gov/27427459/ among others).
Current project: Microcircuitry mapping of V2 and pulvinar inputs to layer 1 of V1 in the macaque.
Techniques: Anterogradely label these two inputs by distinguishable markers, using the recently developed subcellular compartment-specific peroxidase-based electron microscopy labeling (Zhang et al., 2019 https://pubmed.ncbi.nlm.nih.gov/30886406/ ).
Combine with X-ray holographic nano-tomography (XNH) to localize labeled inputs and their postsynaptic distal apical dendrites. Often overlooked is the fact that the population of distal dendrites in V1 and V2 are different. In V1, distal dendrites in layer 1 are from neurons in layers 2, 3, 5, and (selectively) 6. In V2, however, the layer 5 dendrites drop out (Peters et al., 1997; Lund et al., 1981).
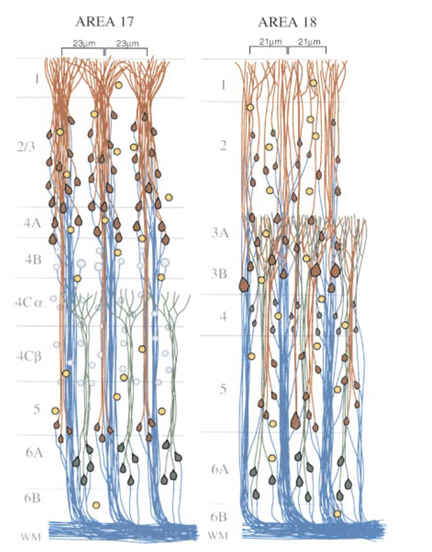
Peters et al., 1997. In V1 (area 17), layer 5 apical dendrites extend into layer 1, but in area V2, apical dendrites of layer 5 neurons (arrow) do not extend beyond layer 3A. As a consequence, for V2 feedback to V1, only the apical dendrites of the supragranular population are directly contacted by feedback from V4 to V2 (and see Lund et al., 1981).
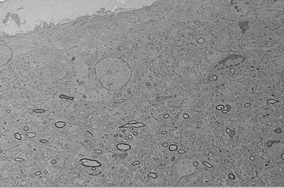
Electron micrograph of layer 1 of V1 (macaque monkey).
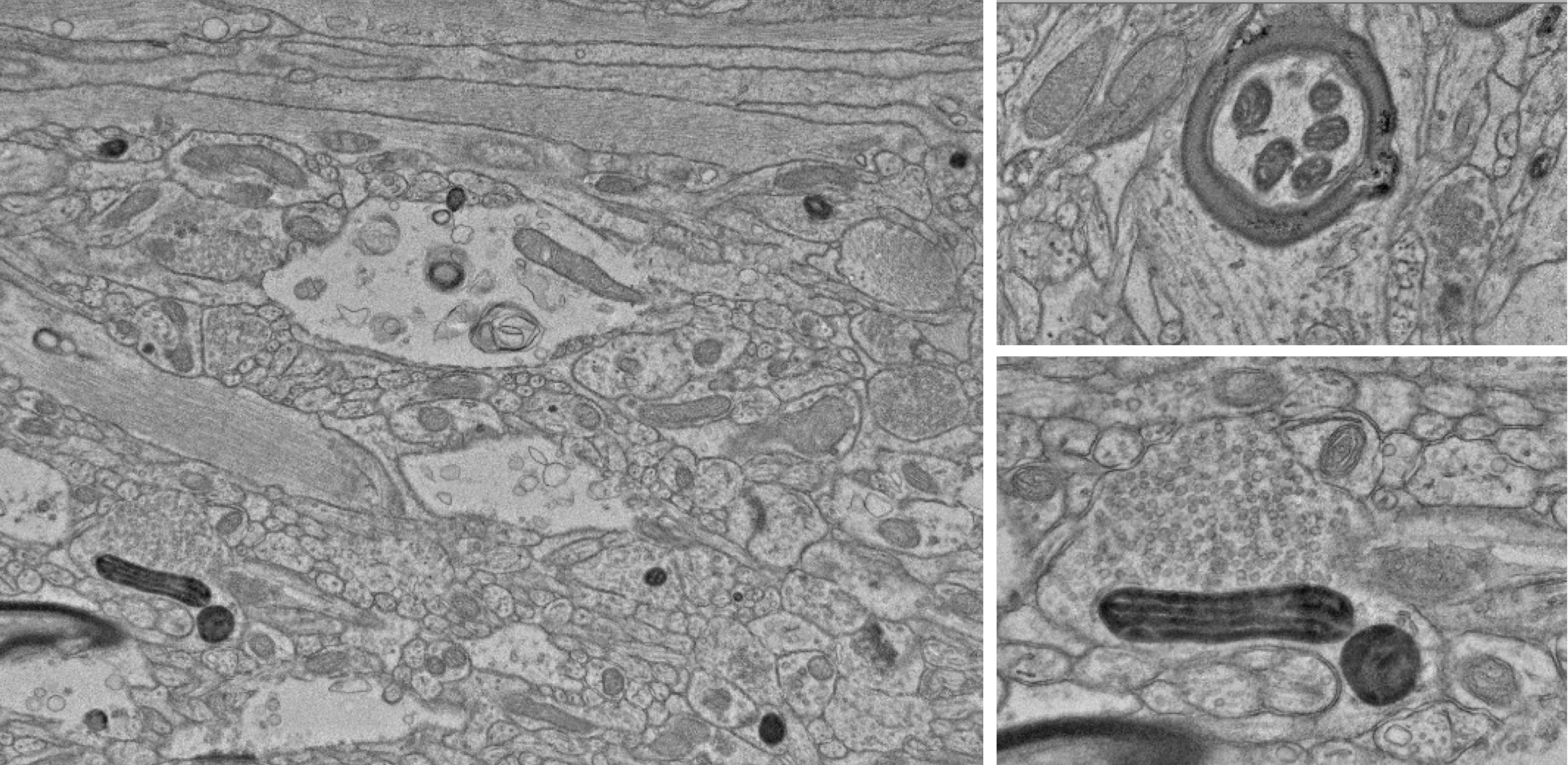
Several profiles in V1 with dAPEX2 labeled mitochondria, after a viral injection in area V2.
Back
Using online databases for anatomically-based research.
Collaborator: Dr. Alvaro Duque (Yale University)

Student trainee: Mitali Sakharkar (BU 6 year BS/MD student)

I. Background: Digital databases are increasingly important, both in the teaching and research domains. This is especially so for the nonhuman primate brain, where building a lab-based anatomical database is necessarily labor intensive and expensive. The availability of the NIH-funded MacBrain database (link above: Collection 6) is thus an important resource. I have been interacting with Dr. Alvaro Duque (PI) on ways to best position this for teaching and research purposes. Materials will be used for AN724 (Spring semester, co-instructor with Dr. Rushmore).
Completed: Neurochemical dissection of the Complex neurochemical microstructure of the stria terminalis in infant and adult macaque monkey. (Sakharkar, Rockland, and Duque, 2022)
As a prototype project, we have characterized the micro-structure of the stria terminalis (ST) in four infant and two adult macaque brains. The ST is a C-shaped fiber bundle, associated with the amygdala and bed nucleus of the stria terminalis (BNST).
As a teaching tool, this has offered opportunity for:
– learning to navigate the NHP brain in coronal tissue sections.
– identifying the ST in its characteristic location medial to the caudate nucleus (tail and body).
As a research tool, this has facilitated several specific results. For example:
– cellular populations, as visualized by NeuN.
– cellular subpopulations, as visualized by antibodies against neuropeptide Y, calretinin, calbindin, and others.
– compartmentalization, with reference to myelin-dense and myelin-sparse regions (visualized by antibodies against myelin basic protein).
– fiber orientation.
Below, location of the ST (ventral component), medial to the caudate nucleus. Coronal section reacted for NeuN, a pan-neuronal marker.
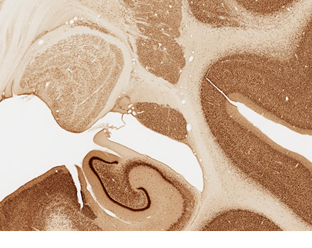
Two coronal sections, spaced 3.0mm apart, reacted for tyrosine hydroxylase (TH). ST is indicated by arrowheads. In the two higher magnification images (below), TH+ fibers are visualized. These travel along the circumference of the ST and concentrate in a beltlike pattern, cutting medial-lateral across the ST.
Back
Computational modeling of diverse cortical neurons and networks involved in working memory.
In collaboration with Christina Weaver (Franklin & Marshall College, Lancaster, PA) and Klaus Wimmer (Centre de Recerca Matemàtica, Barcelona, Spain), we build computational models that predict how alterations observed empirically with aging and neurodegeneration affect neuronal function. Our models operate at various scales, from individual pyramidal neurons, to local cortical networks, and across multiple brain areas, allowing us to understand the complex, nonlinear functions of the brain in new ways. The models are constrained by a wide range of our empirical data in rhesus monkeys and mice: electron and confocal microscopy, immunohistochemistry, electrophysiology, and behavioral testing.
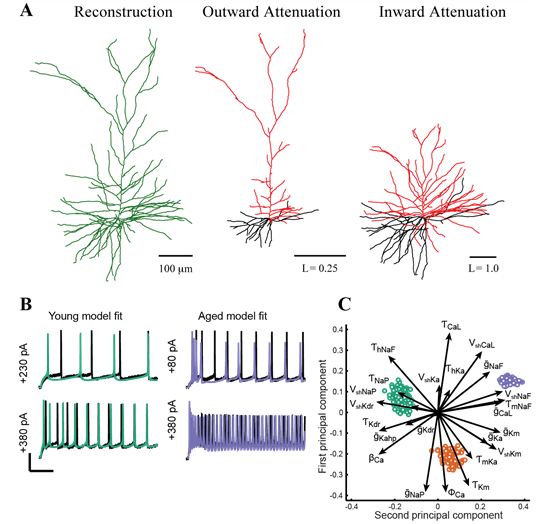
Computational modeling of pyramidal neurons. A: Morphoelectrotonic transforms demonstrate how signals attenuate outward from, or propagating in toward, the soma. Modified from Amatrudo et al (2012). B: Automated optimization enables fits of model parameters so that model outputs (green and blue lines) are similar to empirically measured in vitro voltage responses to current step injections. C: Analysis techniques including principal components analysis predict how individual ion channels may be affected by aging (green: young model population; red and blue, aged model populations). Panels B-C were modified from Rumbell et al. (2016).
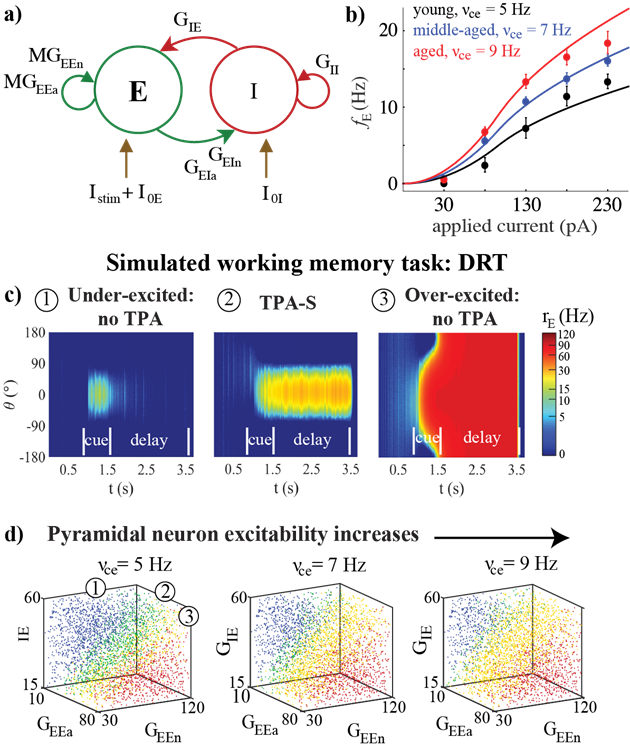
Effects of aging on a network model of the delayed response task (DRT). For details, see Ibañez et al. (2020).
Back
Current Projects
2023 SFN Abstract. High resolution histological definition in the rhesus monkey brain of fiber trajectories (ChAT, TH, and 5HT) accessing the anterior cingulum bundle. Rockland and Duque. (2023 IBRO Abstract 2332)
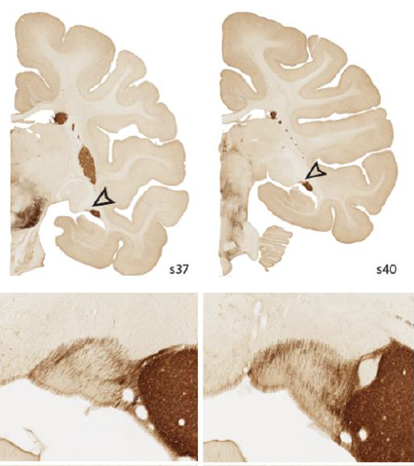
2023 IBRO Abstract 2332. NON MEDIOLATERAL TRAJECTORY OF FIBER POPULATIONS IN THE MACAQUE CORPUS CALLOSUM. Rockland and Duque.


Macaque corpus callosum: Tyrosine hydroxylase positive fibers aggregate in narrow mediol-laterally oriented mini-striae. In coronal sections, these appear as short axon segments in an oblique anterior-posterior orientation Double asterisks indicate the same tissue location at low (left) and higher magnification (right). Brain 79, section 20 from MacBrain Resource Center Collection 6. Neurochemically distinguished subpopulations can be visualized in postmortem human tissue as well.
Lab Members
As of 11/21/21
Kathy Rockland, PhD
Lab Director: Contact
Lab History
Soon after re-joining the Anatomy & Neurobiology department in 2012, I initiated a new project on white matter neurons (“interstitial neurons”) with Drs. Farzad Mortazavi and Doug Rosene, with excellent technical support from Samantha Romano and Andrew Chang (now in the BU MD/PhD program). For current projects, see above.
The previous ten years were my Japan years, at RIKEN Brain Science Institute (BSI; now CBS). Most lab members were Japanese technical staff and postdocs, who have since gone on to academic positions in Japan. We also had a number of international guests and trainees, with many of whom I have been able to keep in close contact. I co-organized a conference in Erice with Elena Borra (University of Parma) in 2016, and recently co-authored a paper with her and her colleagues on white matter neurons (Borra et al., 2020). Michele Pignatelli, who visited from Lausanne in 2008, is now a nearby Cambridge colleague (at MIT), and also a recent co-author with me on a chapter (Development of Hippocampal Circuitry).
Another significant stage in my career was the approximately ten years before, at the University of Iowa, Dept. of Neurology (~1990-2000). This was the core period of my work on single axon reconstruction, when I and a group of excellent technical staff, and postdoctoral fellow Song-lin Ding (now at Allen Brain Institute) and Bryan Wellman (neurosurgery fellow) explored topological and quantitative features of cortical connections, using Golgi-like anterograde labels (PHAL and then biotinylated dextran amine). An important backdrop was the neuropsychology group within the Dept. of Neurology and, on the more anatomical front, Dr. Gary Van Hoesen, another Boston émigré from the wonderful Norman Geschwind group, which included both Drs. Deepak Pandya and Doug Rosene, among others. During the Iowa years, I was also collaborating with Drs. Keiji Tanaka, K.S. Saleem, and Manabu Tanifuji at RIKEN (Japan). This led to the invitation to establish a neuroanatomy systems lab at RIKEN BSI, as described in the previous paragraph.

Erice, Sicily (2017)

2005 Laboratory for Cortical Organization and Systematics (COS), RIKEN BSI
Recent Publications
Castro-Mendoza PB et al. Proteomic features of gray matter layers and superficial white matter of the rhesus monkey neocortex: comparison of prefrontal area 46 and occipital area 17. Brain Structure Function 2024 online ahead of print doi: 10.1007/s00429-024-02819-y.
Rockland KS. Cellular and laminar architecture: A Short history and commentary. J Comp Neurol 2023 Dec 531 (18) 1826-1933. doi: 10.1002/cne.25553.
Rockland KS. A brief sketch cross multiscale and comparative neuroanatomical features. Fron Neuroanat, 2023 Feb 13; 17: 1108363. doi: 10.3389/fnana.2023.1108363.
Rockland KS. Looking for the origins of axons. Elife 2022; June 1 1:e79839. doi: 10.7554/eLife.79839.
Rockland KS. Clustered intrinsic connections: Not a single system. Front Syst Neurosci. 2022 Jun 3;16:910845. doi: 10.3389/fnsys.2022.910845.
DeFelipe J et al. Neuroanatomical and pyschological considerations in temporal lobe epilepsy. Front Neuroanat. 2022 Dec 14;16:995286. doi: 10.3389/fnana.2022.995286.
Contact Us
Rockland Lab
72 East Concord St. Boston, MA 02118
Email: krock@bu.edu