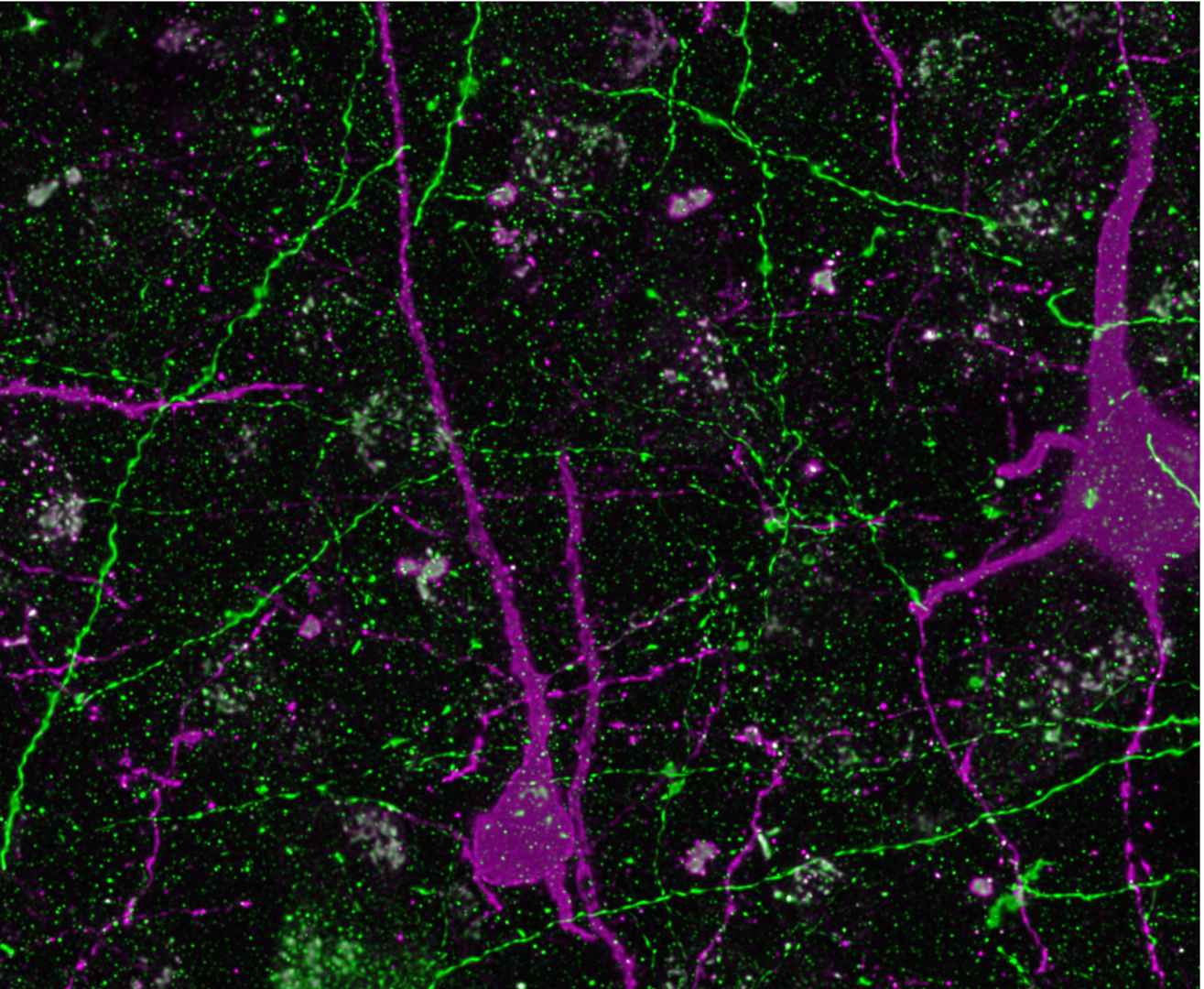
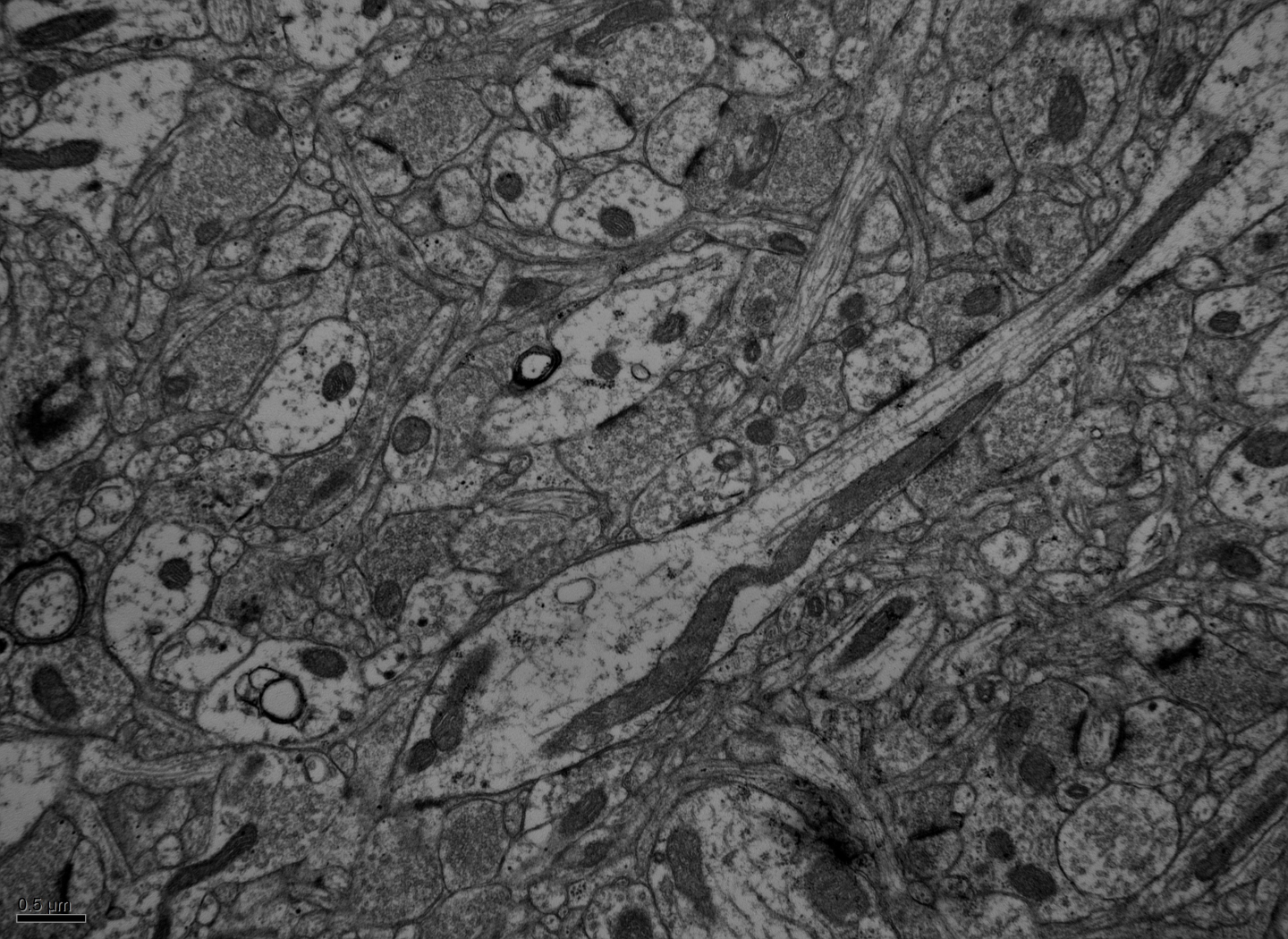
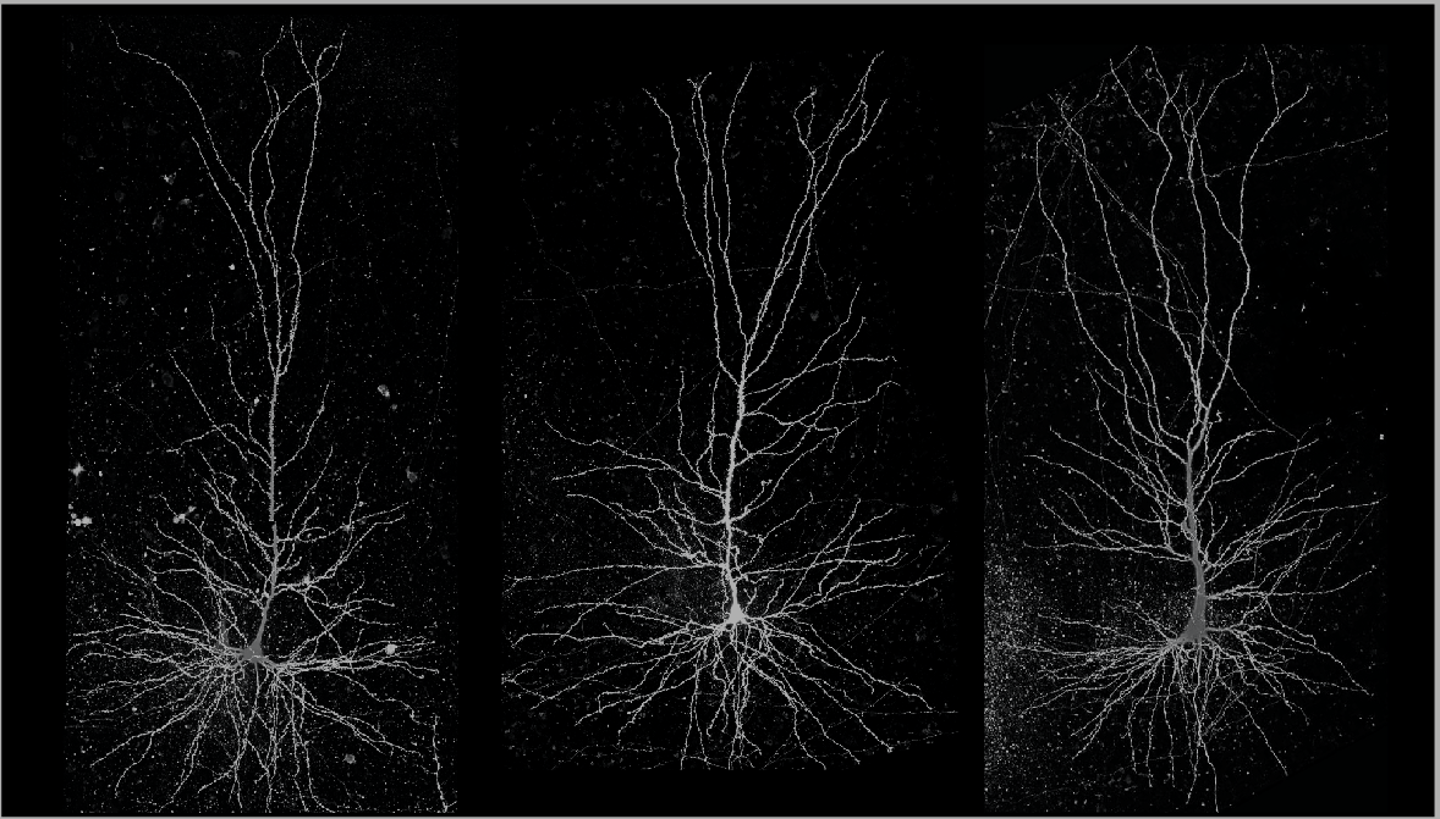
Laboratory of Neural circuits and Ultrastructure
Our Research
The major goal of our work is to understand how distinct limbic, sensory and motor networks interact and are controlled by the lateral prefrontal cortex (LPFC) and the anterior cingulate cortex (ACC) – key areas of the prefrontal “executive” network. Given its dense connections with limbic structures such as the hippocampus and amygdala, the ACC is particularly important for cognitive-emotional integration and is selectively disrupted in many affective disorders such as depression and anxiety.
– How is this prefrontal “cognitive-emotional” network wired?
– Within area microcircuitry
– inter-areal connectivity
– What makes cortico-limbic circuits vulnerable in neuropsychiatric disease?
We combine cellular in vitro electrophysiological methods combined with optogenetic, multi-scale anatomic, and transcriptomic techniques to understand the biophysical and synaptic properties of neurons within functionally-related cortical networks. We utilize whole-cell patch-clamp recording, pathway tract-tracing, multiple immunohistochemical labeling, and 3D imaging techniques for light and electron microscopy (EM). We incorporate empirical data into biophysical network models to predict how diverse single-cell intrinsic and synaptic circuit properties can affect network dynamics.
Pathway tracing of prefrontal long-range connections
Our focus is on the laminar organization and convergence of long-range limbic, motor and cognitive pathways within the anterior cingulate cortex (ACC), which are implicated in cognitive-emotional integration.
Our recent study has found distinct dorsal-motor and ventral-limbic ACC sectors that are integrated by amygdalar inputs (Calderazzo et al., 2021, JCN).
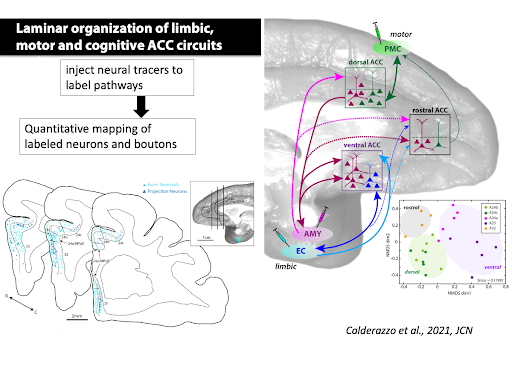
Calderazzo SM, Busch SE, Moore TL, Rosene DL, Medalla M. (2021) Distribution and overlap of entorhinal, premotor, and amygdalar connections in the monkey anterior cingulate cortex. J Comp Neurol. 2021 Mar;529(4):885-904. doi: 10.1002/cne.24986. Epub 2020 Aug 13.
Single-cell biophysical and apical dendritic properties of ACC pyramidal neurons are layer- and target-dependent (Medalla at al., 2021, Cerebral Cortex, in press).
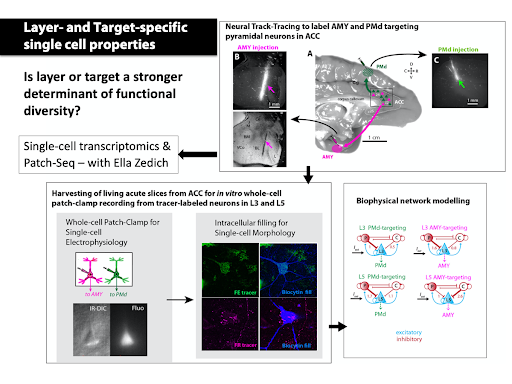
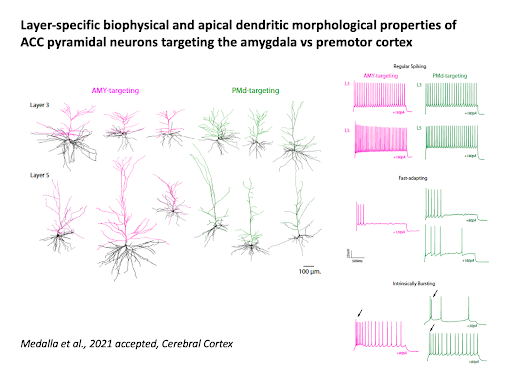
Medalla M, Chang W., Ibañez S., Guillamon-Vivancos T., Nittmann M., Kapitonav A., Busch SE., Moore TL., Rosene DL., Luebke JL (2021). Layer-specific pyramidal neuron properties underlie diverse anterior cingulate cortical motor and limbic networks. Cerebral Cortex, in press
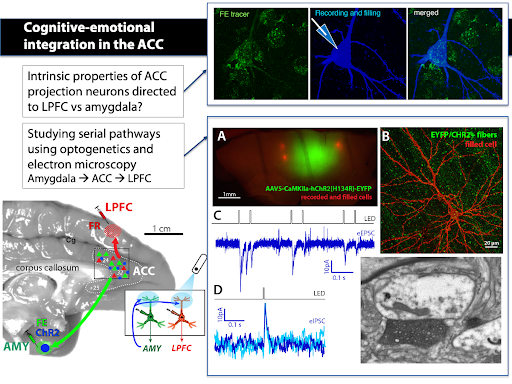
We study pathways interlinking the ACC with the LPFC and the amygdala using retrograde tracing, optogenetics and whole-cell patch clamp recording in in vitro slices. We study synaptic connections using electron microscopy
Back
Regional and Laminar Specialization of prefrontal Excitatory and Inhibitory microcircuits
The laminar organization and diversity of the excitatory and inhibitory microcircuits across primate prefrontal areas are largely unknown. Our work has contributed to an understanding of excitatory and inhibitory synaptic diversity at the single cell level – leading us one step closer to the elusive goal of identifying how distinct pathways are integrated in single neurons.
Our multi-tiered studies using in vitro whole cell, high resolution confocal and electron microscopy and computational model shows that diverse perisomatic inhibitory inputs to ACC and LPFC layer 3 pyramidal neurons confer distinct oscillatory network dynamics in these areas (Medalla et al., 2017 J. Neurosci).
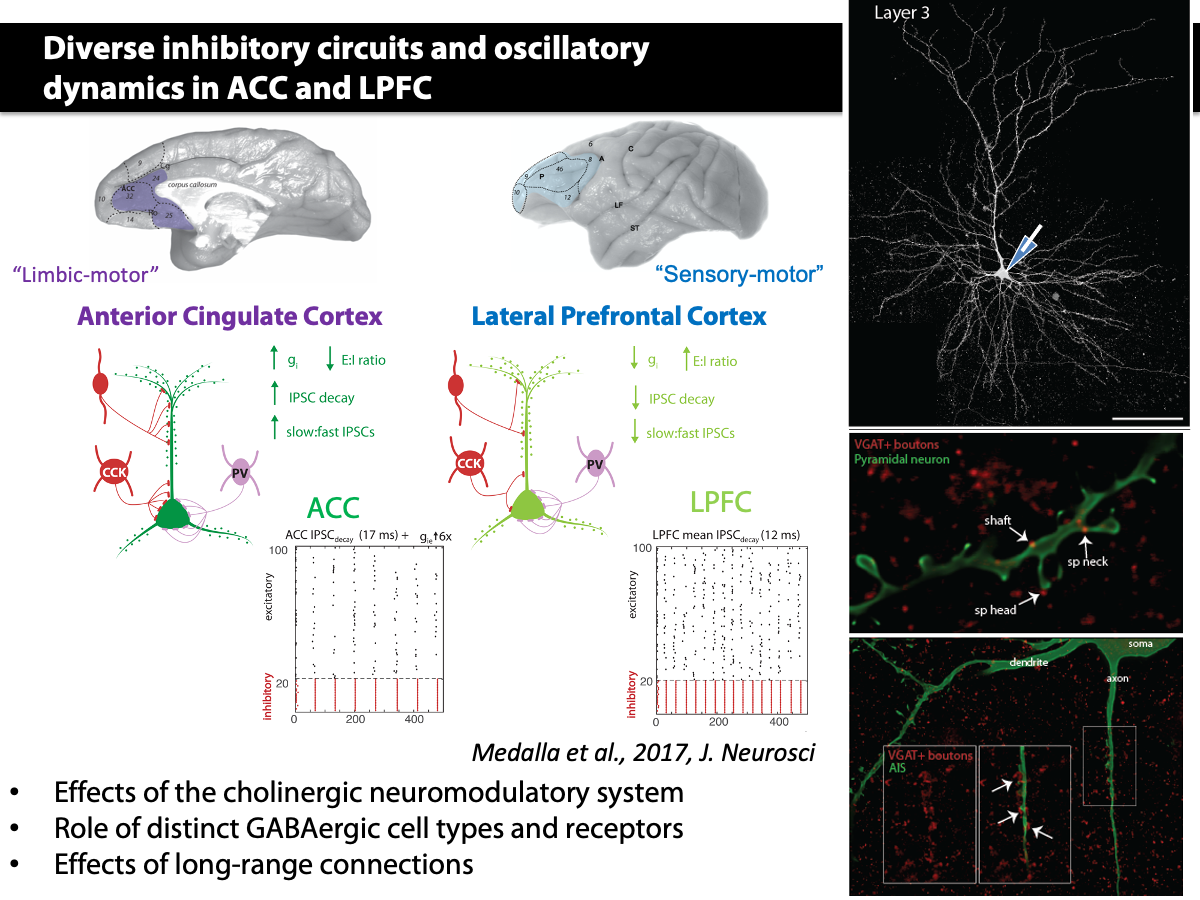
Medalla M, Gilman JP, Wang JY, Luebke JI. Strength and Diversity of Inhibitory Signaling Differentiates Primate Anterior Cingulate from Lateral Prefrontal Cortex. J Neurosci. 2017 May 3;37(18):4717-4734. doi: 10.1523/JNEUROSCI.3757-16.2017. Epub 2017 Apr 5.
In collaboration with Jennifer Luebke, we have shown that in contrast to the cellular and synaptic diversity across monkey cortical areas, visual and frontal areas in the mouse have largely similar cellular and synaptic features.
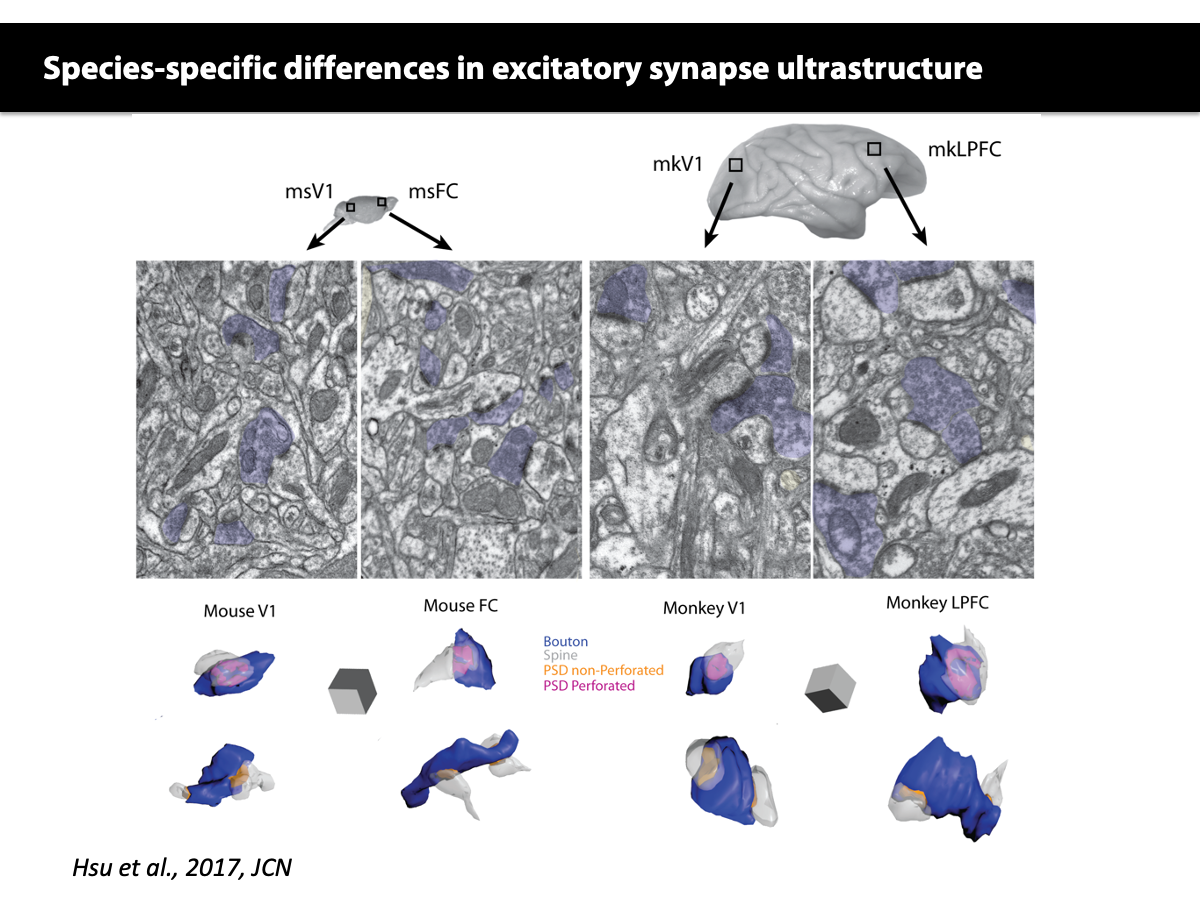
Hsu A, Luebke JI, Medalla M. Comparative ultrastructural features of excitatory synapses in the visual and frontal cortices of the adult mouse and monkey. J Comp Neurol. 2017 Jun 15;525(9):2175-2191. doi: 10.1002/cne.24196. Epub 2017 Mar 26.
Gilman JP*, Medalla M*, Luebke JI. (2016) Area-Specific Features of Pyramidal Neurons-a Comparative Study in Mouse and Rhesus Monkey. Cereb Cortex. 2016 Mar 10. pii: bhw062. [Epub ahead of print] PMID: 26965903 *co-first authors
Medalla M and Luebke, JI. (2015) Diversity of glutamatergic synaptic strength in lateral prefrontal versus primary visual cortices in the rhesus monkey. J Neurosci, 2015 Jan 7; 35(1):112-27. doi: 10.1523/JNEUROSCI.3426-14.2015. PMID: 25568107
In collaboration with Chand Chandrasekaran, we have begun to bridge the gap of laminar structure, connectivity and dynamics of task-relevant single cell activity in monkeys:
Lee EK, Balasubramanian H, Tsolias A, Anakwe SU, Medalla M, Shenoy KV, Chandrasekaran C (2021). Non-linear dimensionality reduction on extracellular waveforms reveals cell type diversity in premotor cortex. Elife. 2021 Aug 6;10:e67490. doi: 10.7554/eLife.67490. Online ahead of print.
In collaboration with Dr. Luebke and Chandrasekaran, we will use viral tracing and optogenetics of specific excitatory and inhibitory cell types to further probe these questions.
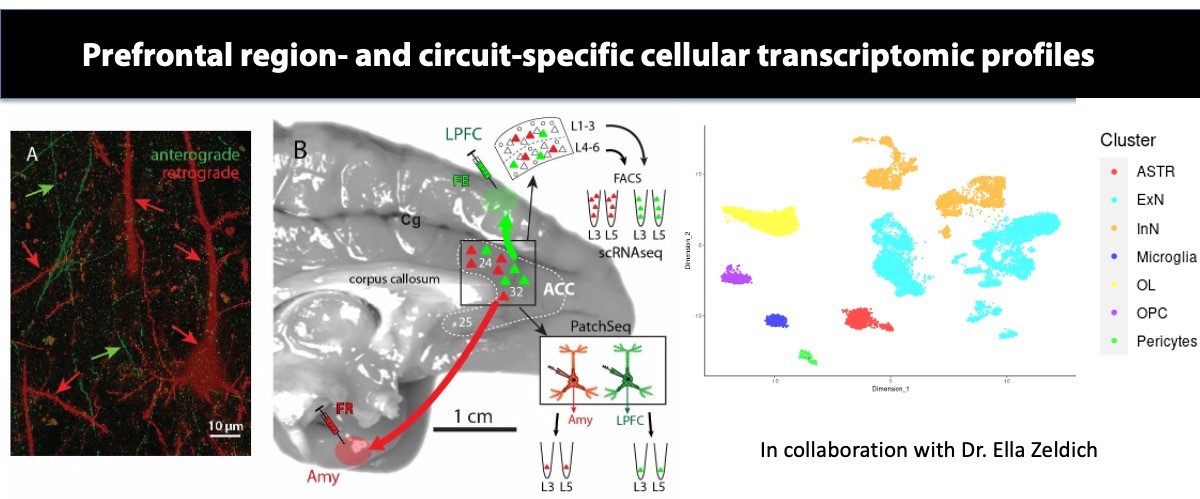
In collaboration with Dr. Ella Zeldich, we will use state-of-the-art single-cell RNAseq transcriptomics combined with tract-tracing and in vitro electrophysiological techniques, to determine the molecular signature, neurochemical and functional profile of lamina-specific prefrontal excitatory, and inhibitory neurons that control arousal and stress. The proposed study will unravel the molecular underpinnings of laminar and functional diversity of ACC circuits mediating affective behavior, which has broad therapeutic implications for understanding neurochemical imbalance and susceptibility in stress-related disorders.
Back
Circuit Plasticity in Aging, Injury, and Neurodegeneration
My collaborative work has explored the cellular changes that occur with aging and after injury in the frontal cortex of monkeys, and selective tau neuropathology in medial temporal areas in rodents.
In ongoing projects with Drs. Tara Moore and Douglas Rosene, we have found that injury in motor cortices leads to neuronal hyperexcitability, microglial inflammation, and dysregulation of oligodendrocyte and myelin plasticity. Further, in collaboration with Drs. Ben Buller and Hongqi Xin from Henry Ford, we have shown that these injury-related pathological changes are ameliorated by systemic treatment with extracellular vesicles (EV) derived from bone-marrow stem-cells, acting as immune-modulators that can stimulate neural plasticity after injury.
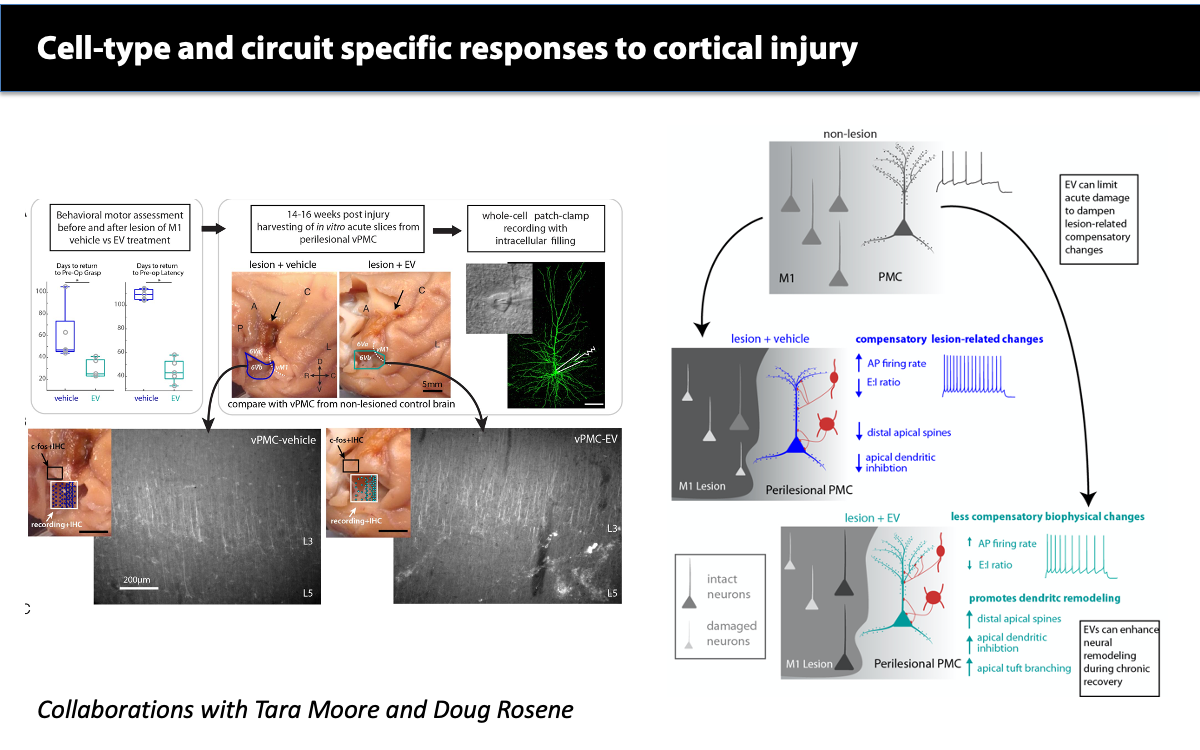
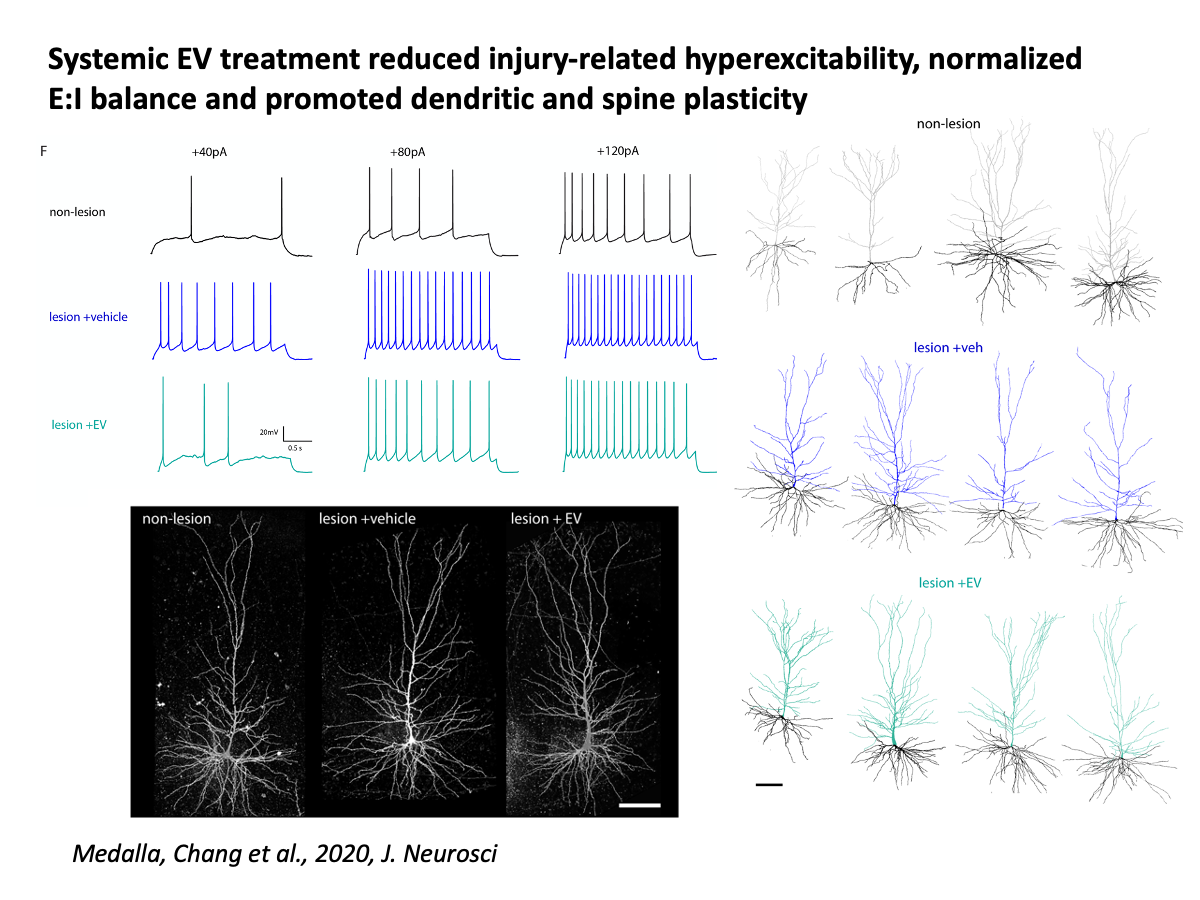
Medalla M, Chang W, Calderazzo SM, Go V, Tsolias A, Goodliffe JW, Pathak D, De Alba D, Pessina M, Rosene DL, Buller B, Moore TL. (2020) Treatment with Mesenchymal-Derived Extracellular Vesicles Reduces Injury-Related Pathology in Pyramidal Neurons of Monkey Perilesional Ventral Premotor Cortex. J Neurosci. 40(17):3385-3407. doi: 10.1523/JNEUROSCI.2226-19.2020. Epub 2020 Apr 2.
Go V, Sarikaya D, Zhou Y, Bowley BGE, Pessina MA, Rosene DL, Zhang ZG, Chopp M, Finklestein SP, Medalla M, Buller B, Moore TL. (2020) Extracellular vesicles derived from bone marrow mesenchymal stem cells enhance myelin maintenance after cortical injury in aged rhesus monkeys. Exp Neurol.:113540. doi: 10.1016/j.expneurol.2020.113540. Online ahead of print.
Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL. (2020) Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience. 2020 Feb;42(1):1-17. doi: 10.1007/s11357-019-00115-w. Epub 2019 Nov 6.
Moore TL, Bowley BGE, Pessina MA, Calderazzo SM, Medalla M, Go V, Zhang ZG, Chopp M, Finklestein S, Harbaugh AG, Rosene DL, Buller B. (2019) Mesenchymal derived exosomes enhance recovery of motor function in a monkey model of cortical injury. Restor Neurol Neurosci. 2019;37(4):347-362. doi: 10.3233/RNN-190910.
My collaborative studies in mice, extend these questions of neuronal survival and degeneration in the context of mouse models of tauopathy. With Dr. Tsuneya Ikezu, we have investigated mechanisms of tau propagation through the entorhinal cortex and hippocampal pathways. With Dr. Ben Wolozin, we have investigated the role of RNA-binding proteins in the neurodegenerative and inflammatory progression of tau pathology within this medial temporal network. These important findings begin to address the question of what allows for some neurons to survive and others to degenerate in disease and injury.
Ash PEA, Lei S, Shattuck J, Boudeau S, Carlomagno Y, Medalla M, Mashimo BL, Socorro G, Al-Mohanna LFA, Jiang L, Öztürk MM, Knobel M, Ivanov P, Petrucelli L, S Wegmann S, Kanaan NM, Wolozin B (2021) TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau. Proc Natl Acad Sci U S A. 2021 Mar 2;118(9):e2014188118. doi: 10.1073/pnas.2014188118.
Muraoka S, DeLeo AM, Sethi MK, Yukawa-Takamatsu K, Yang Z, Ko J, Hogan JD, Ruan Z, You Y, Wang Y, Medalla M, Ikezu S, Chen M, Xia W, Gorantla S, Gendelman HE, Issadore D, Zaia J, Ikezu T. (2020) Proteomic and biological profiling of extracellular vesicles from Alzheimer’s disease human brain tissues. Alzheimers Dement. 2020 Jun;16(6):896-907. doi: 10.1002/alz.12089. Epub 2020 Apr 17.
LeBlang CJ, Medalla M, Nicoletti NW, Hays EC, Zhao J, Shattuck J, Cruz AL, Wolozin B, Luebke JI. (2020). Reduction of the RNA Binding Protein TIA1 Exacerbates Neuroinflammation in Tauopathy. Front Neurosci. 14:285. doi: 10.3389/fnins.2020.00285. eCollection 2020.
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke JI, Haydar T, Wolozin B, Butovsky O, Kügler S & Ikezu T (2015), Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015 Oct 5. doi: 10.1038/nn.4132. [Epub ahead of print]. PMID: 26436904
Back
Lab Members
As of 02/13/22
Maya Medalla, PhD
PI: Contact
Jennifer Luebke, PhD
PI – Collaborator: Contact
Lorenze Ponce
Lab Manager: Contact
Mary Kate Joyce, PhD
Post-Doc: Contact
Chrome Mojica
PhD Student: Contact
Yuxin Zhou
PhD Student: Contact
Alumni
As of 02/17/22
| PhD Students | |
|---|---|
| Alexandra Tsolias | (A&N) |
| Wayne Chang | (A&N) |
| Master’s Students and Employees | |
| Bing Mo | (A&N) |
| Hrishti Bhatt | (A&N) |
| Dickson Chen | (A&N) |
| Junwoo Louis Park | (A&N) |
| Rakin Nasar | (A&N) |
| Mathias Nittmann | (MAMs) |
| Charles Kopp | (MAMs) |
| Samantha Calderazzo | (A&N) |
| Jingyi Wang | (A&N) |
| Joshua Gilman | (MAMs) |
| Silas Busch | (lab technician) |
| Alexander Hsu | (MS A&N, & lab technician) |
| Undergraduate Research Students | |
| Chantal Aaron | (BU PREP student) |
| Diego DeAlba | (STaRs and BU PREP) |
| Anastasia Kapitonava | (UROP, Undergraduate Thesis student) |
| James Zhao | (UROP) |
| Heejoo Kang | (UROP) |
| Maxine Hsiung | (UROP) |
| Mitali Sakharkar | (UROP) |
| Undergraduate Summer Program Students | |
| Shereeen Abdulkabir | (BU Prep) |
| Lazaro Fernandez | (StaRs) |
| Gerardo Sequen Rivera | (StaRs) |
| Mollie Sherman | (SPIN) |
| Caroline Beneville | (SPIN) |
| Haeji Chung,Courtney Dunphy | (SPIN) |
| Joy Yang | (SPIN) |
| Alexandra Morquette | (StaRs) |
| High School Interns | |
| Marianna Tsolias | (2020-2021) |
| William Alano | (summer 2019) |
| Adrian Lin | (summer 2021) |
Recent Publications
Contact Us
Medalla Lab
650 Albany Street, X306
Boston Massachusetts, 02118
Office Phone: 617-358-7717
Main Lab Phone: 617-358-7721
EM Lab: R1010
Email: mmedalla@bu.edu