Laboratory of Cellular Neurobiology
Our Research
We aim to understand how specific features of neurons and neuropil in diverse cortical regions enable area-specific functionality and how distinctive features may be associated with differential vulnerability in normal and pathological aging.
Together with our collaborators, we employ multifaceted approaches -including single cell NucSeq, PatchSeq, whole-cell patch clamp recording, conventional and multiplexed immunohistochemistry, 2D and 3D electron microscopy, magnetic resonance imaging, behavioral assessments and computational modeling- to quantitatively characterize diverse cortical regions in the mouse and rhesus monkey.
Current major projects include:
1) Age-related changes to cortical dynamics underlying working memory (R01)
2) Mechanisms of age-related cognitive decline in the rhesus monkey (R01)
3) Substrates of selective neuronal vulnerability in aging: neocortical pyramidal neurons and
their surrounding neuropil environment in visual versus frontal cortex of young and aged rhesus monkeys (R21)
Diversity and selective vulnerability of cortical neurons across the adult lifespan.
We study the normative properties of neurons and neuropil in diverse cortical regions to gain insight into how distinct regions accomplish their area-specific functions. We also characterize changes to neurons, glia and vasculature in the prefrontal cortex and visual cortex during normal aging to gain insight into selective neuronal vulnerability and neural substrates of age-related cognitive decline in the primate.
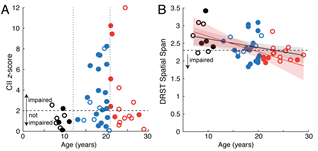
Cognitive changes across the lifespan of rhesus monkeys. A) Cumulative impairment index (CII) z- scores vs. age. open= female; closed= male. B) Performance on the DRST spatial task. Behavioral characterization by Tara Moore, PhD and Doug Rosene, PhD.
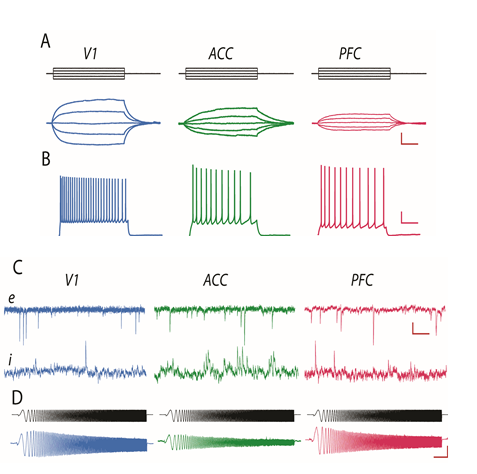
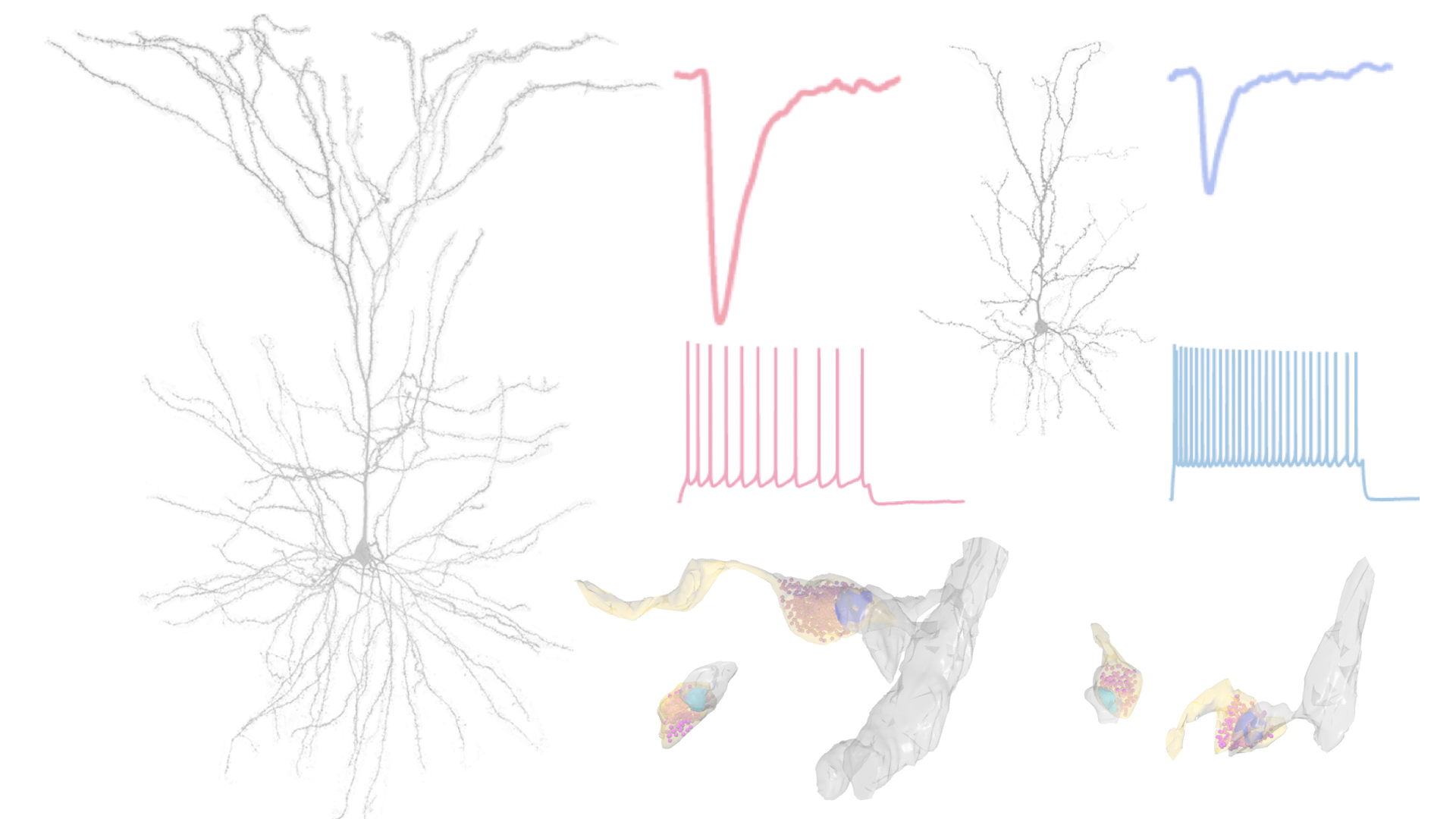
Distinct physiological properties of L3 pyramidal cells in V1, ACC and LPFC. A) Voltage responses of representative cells to a family of current steps (top); input resistance is highest in V1 cells and lowest in LPFC cells. B) Action potentials evoked by a 2s +80 pA current step; V1 cells fire at significantly higher rates than ACC and LPFC cells. C) Top: spontaneous EPSCs; frequency of events is similar in the cells from the 3 areas, their amplitude is lower and kinetics faster in V1 compared to ACC and LPFC cells. Bottom: spontaneous IPSCs demonstrating that the amplitude and kinetics are similar across areas but the frequency of events is dramatically higher in ACC than in V1 or LPFC cells. D) Subthreshold resonance evoked by a CHIRP-ZAP protocol (top); V1 and LPFC cells demonstrate very low frequency (1-2 Hz) resonance while ACC cells do not exhibit significant resonance. Scale bars: A 5mV/50ms; B 10mV/500ms; C 10pA/10ms; D 5mV/1s.
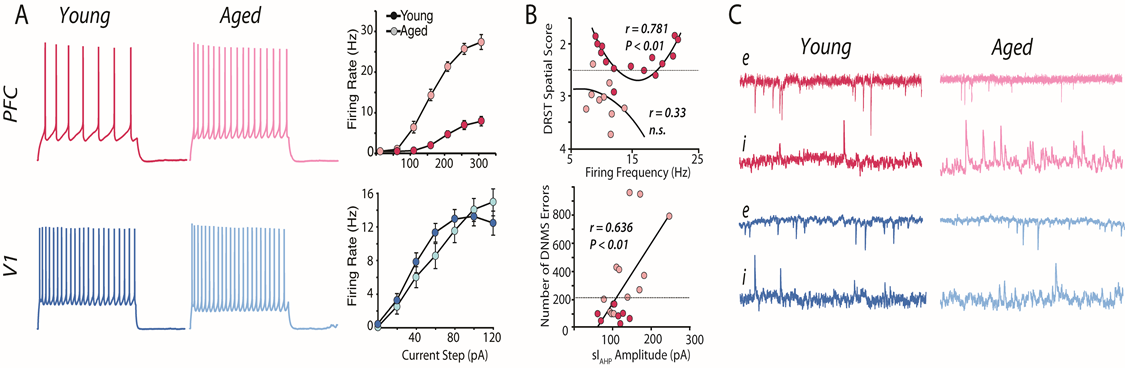
Distinct effects of normal aging on LPFC and V1 neurons. A) Evoked firing rates are significantly increased with age in LPFC (top) but not V1 (bottom) neurons. Mean FR-I graphs showing leftward shift with age in LPFC but not V1 neurons. B) Top, significant U-shaped relationship between mean firing rate of LPFC neurons for a given monkey and that monkey’s performance on the DRST task in aged (dark red) but not young (light red) monkeys. Monkeys above the dashed line (score less than 2.5) are impaired. Bottom, Linear relationship between sAHP current amplitude and number of errors on the DNMS task. C) Top: the frequency of sEPSCs is reduced while that of sIPSCs is increased in aged LPFC neurons cells; Bottom, synaptic events do not change with age in V1 neurons.
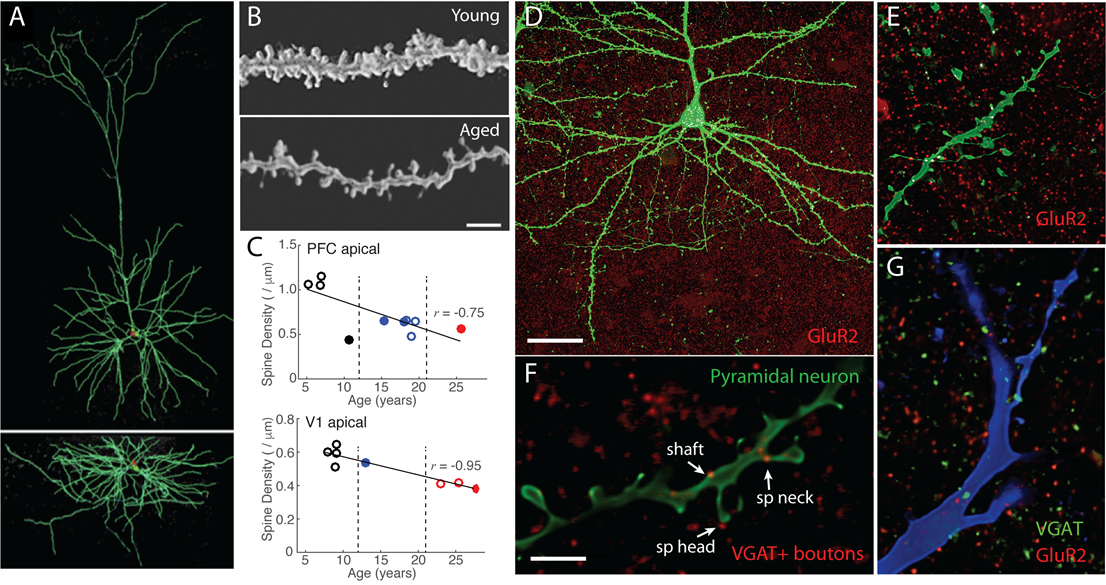
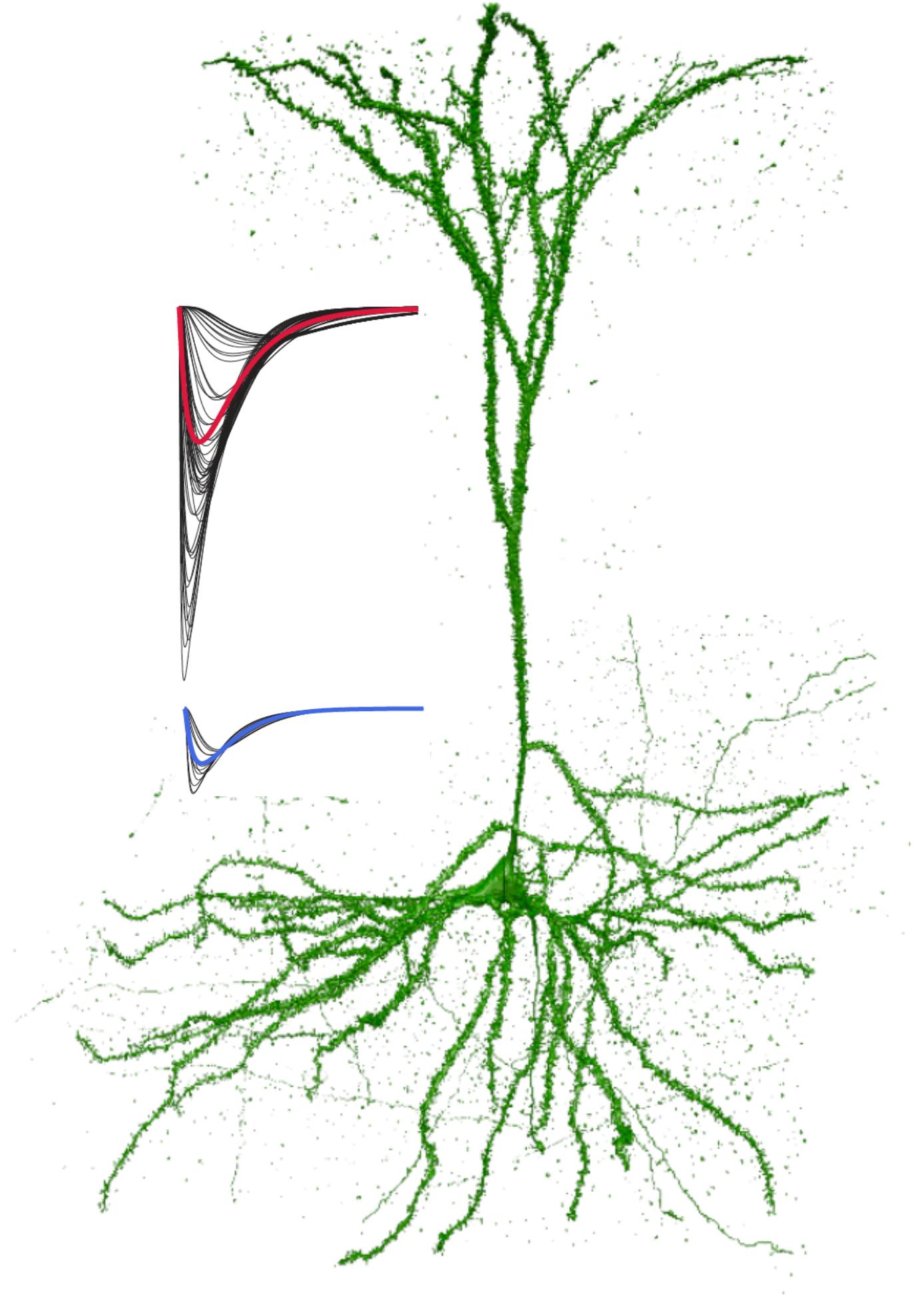
Single neuron morphology and immunohistochemistry. A) Reconstructed L3 LPFC pyramidal cell from a 30-year-old monkey. B) 100x images showing dramatic spine loss in aged LPFC neurons. C) Loss of spines from PFC (top) and V1 (bottom) pyramidal neurons with aging (open=female, closed=male). D) Low and E) high magnification images of GluR2 immunostaining on L3 pyramidal cells. Areas of colocalization between puncta and the filled neuron are shown in white. F) Double immunohistochemistry for VGAT (green) and GluR2 (red) show inhibitory and excitatory synapses in the vicinity of a filled dendrite (blue). G) VGAT+ appositions on dendritic spines and shaft on an LPFC L3 pyramidal cells from a 26-year-old monkey.
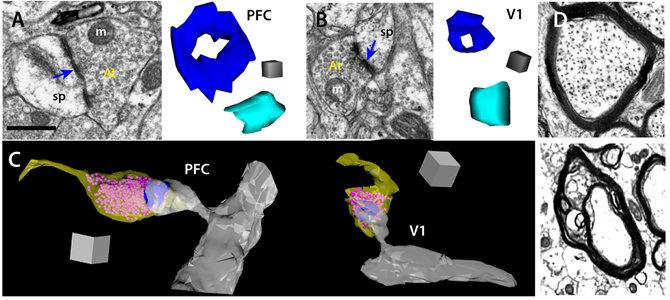
Ultrastructure of synapses and axons. A) Left, EM image of a perforated synapse in LPFC; right, 3D reconstruction of typical perforated (dark blue) and non-perforated (light blue) synapses; m, mitochondria, sp, spine, At, axon terminal. B) Similar synapses shown in 2D and 3D for typical V1 synapses. C) 3D reconstructions of perforated synapses in LPFC vs. V1; yellow- bouton, pink- vesicles, blue- postsynaptic density, gray- spine and dendritic shaft. D) Top, normal myelinated axon in the neuropil of a young compared to a typical dystrophic myelinated axon in an aged monkey (bottom). A,B, bar 0.5 µm; cube 0.05 µm3, C, cube 0.5 µm3. Electron microscopy and ultrastructural analyses performed by Maya Medalla, PhD.
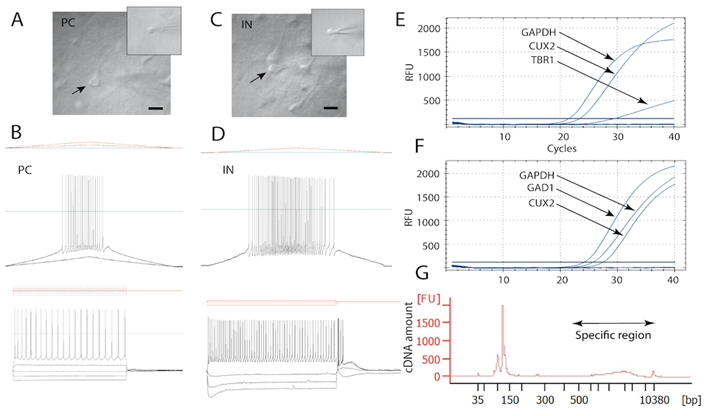
Patch-Seq of L3 pyramidal cells and PV interneurons. Quality validation via Bioanalyzer of the cDNA obtained from a representative harvested L3 pyramidal cell and a fast-spiking interneuron. A, C) IR-DIC images showing harvesting of a pyramidal cell and interneuron respectively in acute slices (arrow), and the somata of these cells collected in the glass pipette (insets). B, D) Firing responses of neurons in A and C to a current ramp stimulus (top) and a series of hyperpolarizing and depolarizing current injections (bottom). E, F) qPCR amplification curves showing expression of housekeeping gene (GAPDPH), neuronal marker for layer 3 (CUX2), and markers specific to pyramidal neurons (TBR1) and inhibitory interneurons (GAD1). G) Quality control validation via Bioanalyzer of the cDNA obtained from a representative harvested L3 pyramida l cell. Arrowhead in D lowest panel indicates the depolarizing “sag” that is due to activation of HCN channels in the interneuron. Patch-Seq performed in collaboration with Ella Zeldich, PhD
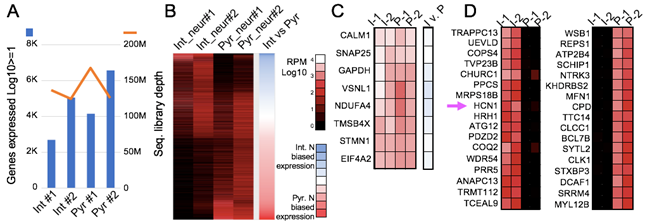
Patch-Seq analysis of 2 interneurons and 2 pyramidal neurons from monkey prefrontal cortex. A) Sequencing and gene mapping statistics. B) Over 9.1K total genes shown in this heat map, grouping genes by neuronal cell-type specific expression. C) Even house-keeping gene expression coverage. D) Top genes exclusively expressed in monkey interneurons (I) and pyramidal neurons (P). Arrow highlights high expression of HCN1 in interneurons but not pyramidal cells. Analyses done in collaboration with Nelson Lau, PhD
Back
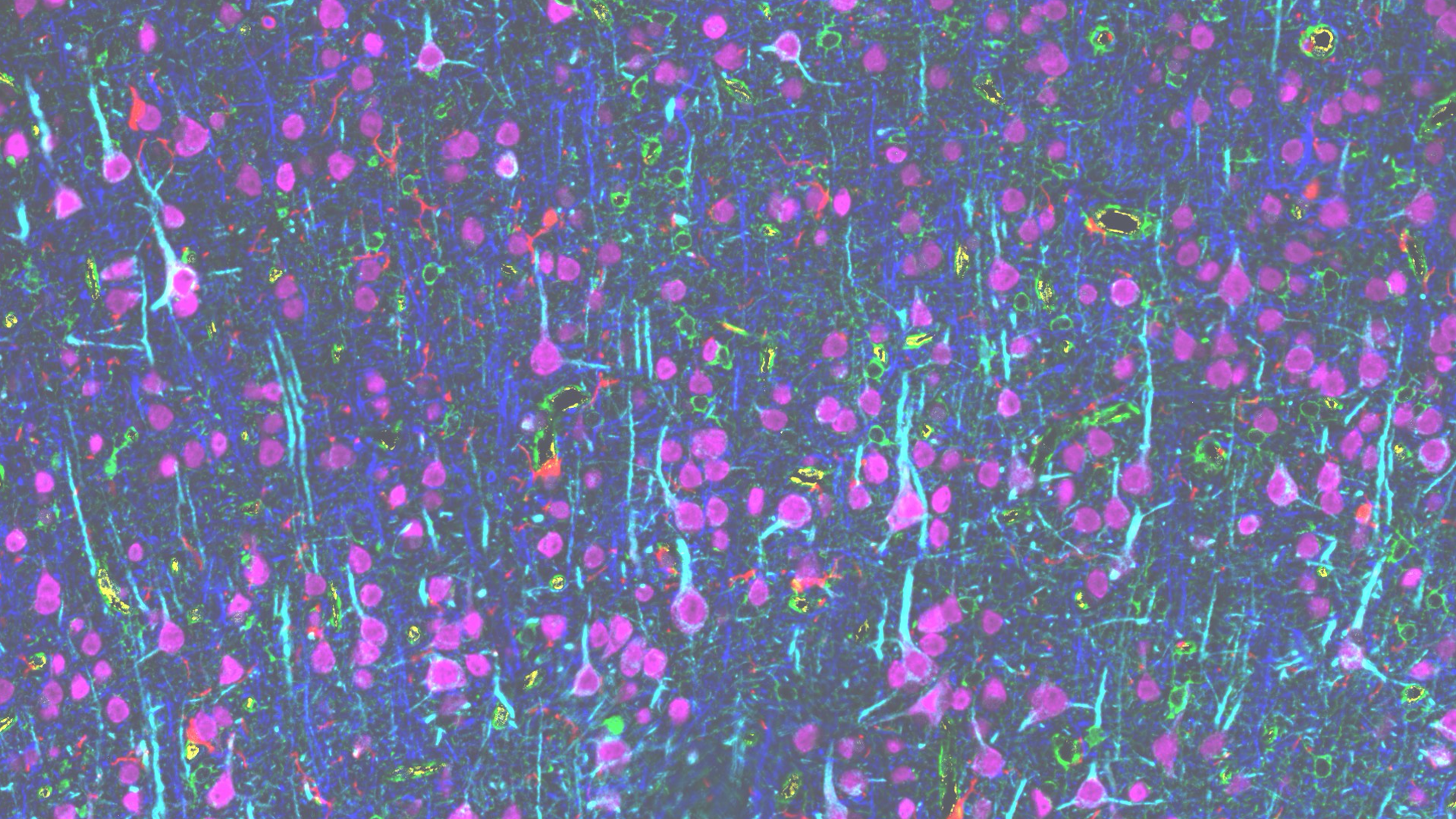
Multiplexed immunohistochemistry assessment of diverse brain areas across the adult lifespan.
In collaboration with Dan Meyer (General Electric) and Patrick Hof (Icahn School of Medicine at Mount Sinai) we employ novel multiplexed immunohistochemistry and high resolution structural analyses of physiologically-characterized individual neurons to compare the properties of individual prefrontal and visual pyramidal neurons in the context of their surrounding neuropil in young and aged rhesus monkeys.
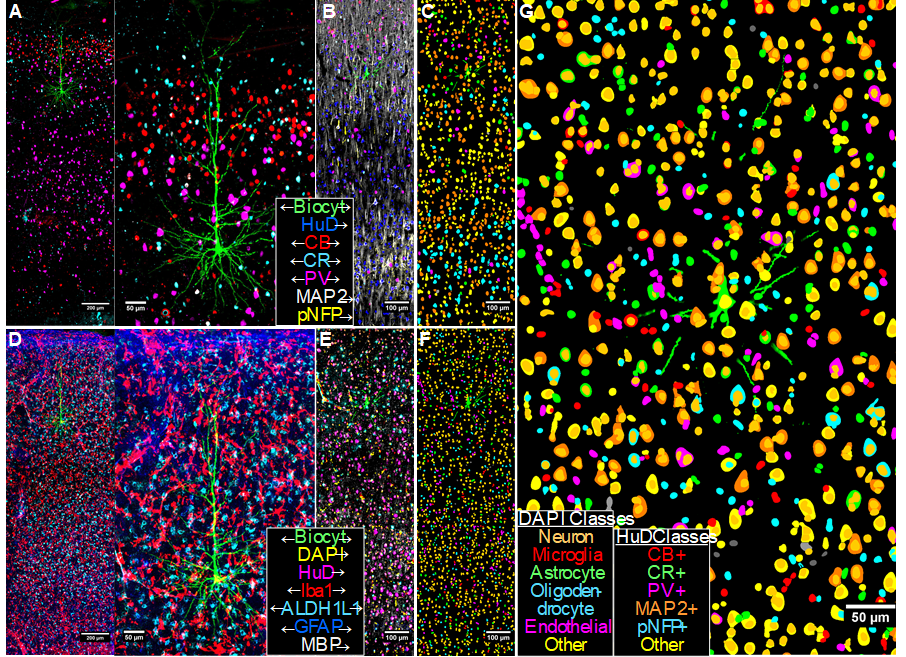
Multiplexed immunofluorescence results for a biocytin-filled L3 LPFC pyramidal neuron. A, D) Maximum intensity projections of 14 3D-aligned, sequential tissue sections for the biocytin channel and select neuronal (A) and glial (D) markers. B, E) Corresponding 2D composite intensity images from a single section, showing additional markers used for machine-learning-based classification of segmented objects-of-interest. C, F) Classification results for HuD-based neuronal soma segmentations (C) and for DAPI-based nuclear segmentations (F). G) Combined overlays of both neuronal and nuclear classifications. The classification object database provides a comprehensive representation of cellular and structural objects, allowing quantitative characterization of class features and spatial detail of the neuropil surrounding the target neuron.
Back
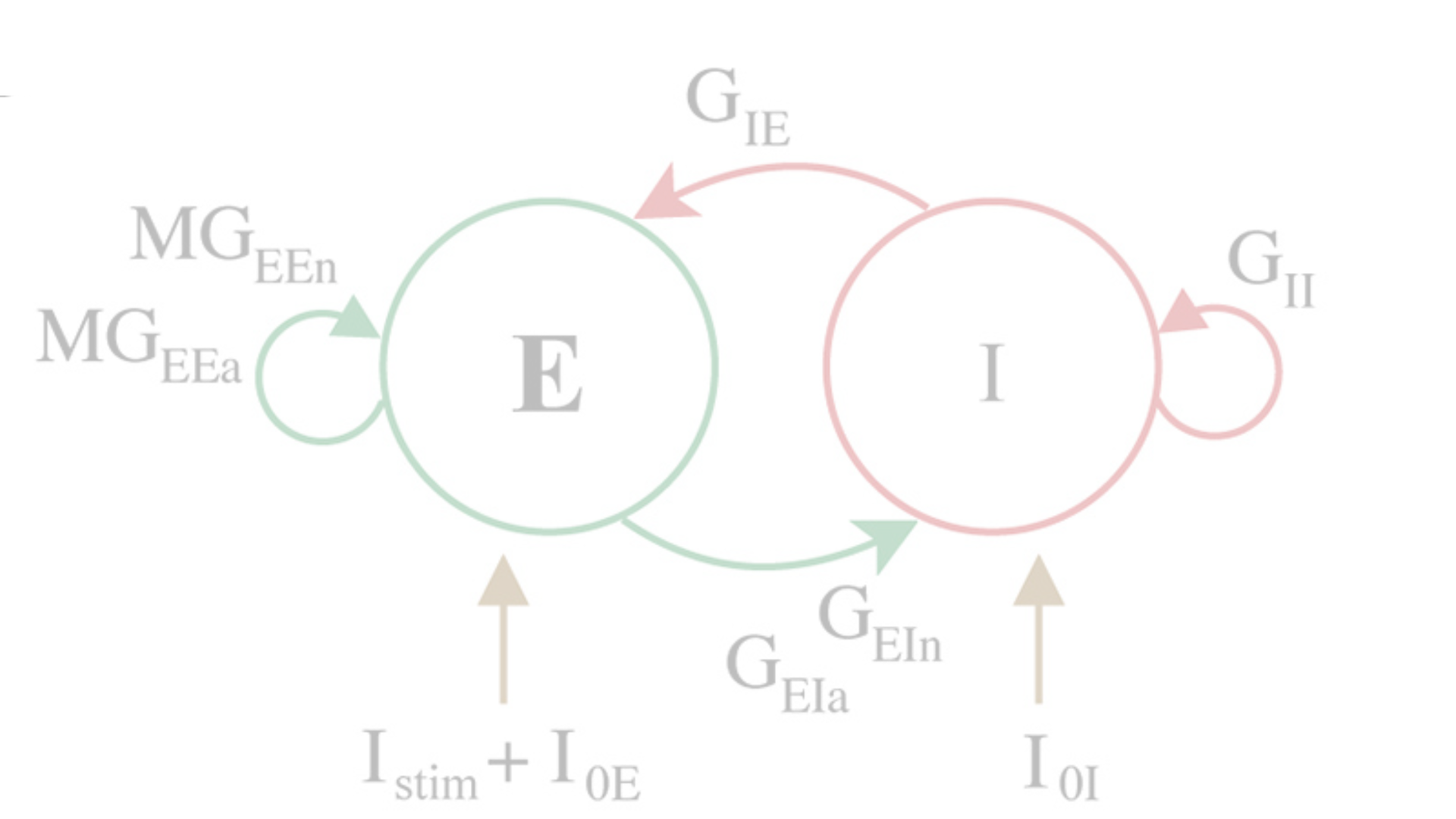
Computational modeling of diverse cortical neurons and networks involved in working memory.
In collaboration with Christina Weaver (Franklin & Marshall College, Lancaster, PA) and Klaus Wimmer (Centre de Recerca Matemàtica, Barcelona, Spain), we build computational models that predict how alterations observed empirically with aging and neurodegeneration affect neuronal function. Our models operate at various scales, from individual pyramidal neurons, to local cortical networks, and across multiple brain areas, allowing us to understand the complex, nonlinear functions of the brain in new ways. The models are constrained by a wide range of our empirical data in rhesus monkeys and mice: electron and confocal microscopy, immunohistochemistry, electrophysiology, and behavioral testing.
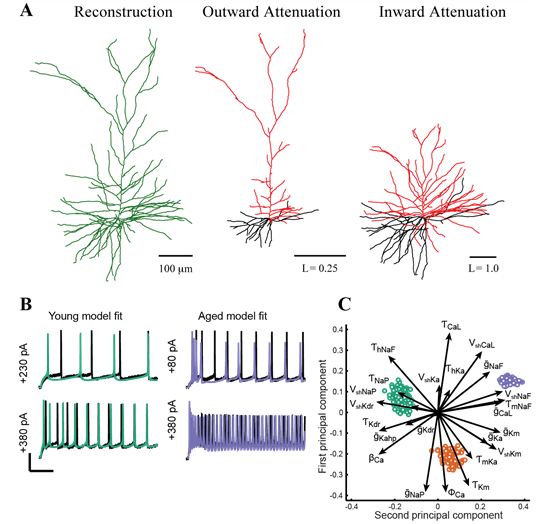
Computational modeling of pyramidal neurons. A: Morphoelectrotonic transforms demonstrate how signals attenuate outward from, or propagating in toward, the soma. Modified from Amatrudo et al (2012). B: Automated optimization enables fits of model parameters so that model outputs (green and blue lines) are similar to empirically measured in vitro voltage responses to current step injections. C: Analysis techniques including principal components analysis predict how individual ion channels may be affected by aging (green: young model population; red and blue, aged model populations). Panels B-C were modified from Rumbell et al. (2016).
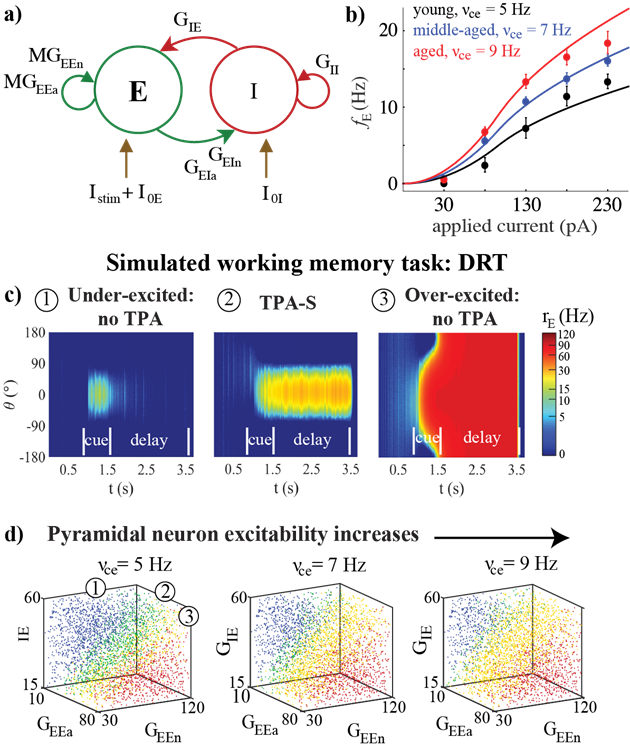
Effects of aging on a network model of the delayed response task (DRT). For details, see Ibañez et al. (2020).
Back
Lab Members
As of 02/17/21
Jennifer Luebke, PhD
Lab Director: Contact
Maya Medalla, PhD
Key Collaborator: Contact
Chrome Mojica
Lab Manager: Contact
Paola Castro-Mendoza
Lab Technician: Contact
Yuxin Zhou
PhD Student (Medalla Lab): Contact
Ian More
Postdoc: Contact
Nilapratim Sengupta
Postdoc: Contact
Alumni
As of 02/17/21
| Postdoctoral Trainees | |
|---|---|
| Dhruba Pathak, PhD | 09/2017- 8/2020 |
| Joe Goodliffe, PhD | 1/2016- 6/2019 |
| Maria Medalla, PhD | 3/2012-7/2015 |
| Katie Youmans PhD | 5/2011-5/2015 |
| Anne Rocher, PhD | 11/2006-4/2010 |
| James Nilson, MD, PhD | 5/2005-1/2007 |
| Doctoral Trainees | |
|---|---|
| Wayne Chang | |
| Chelsey Leblang | 5/2016-5/2020 |
| Teresa Guillamon-Vivancos | 8/2012-7/2017 |
| Johanna Crimins | 9/2008-5/2013 |
| Joseph Amatrudo | 9/2008-1/2013 |
| Kathy Kopeikina (Co-Advisor) | 9/2008-5/2012 |
| Yu-Ming Chang | 8/2001-5/2005 |
| Jason Kass (Co-Advisor) | 1/2001-5/2007 |
| Masters Trainees (since 2015) | |
|---|---|
| Bingxin Mo | |
| Paola Castro-Mendoza | 5/2020-Present |
| Dickson Chen | 5/2020-5/2021 |
| Rakin Naser | 5/2019-5/2020 |
| Junwoo Park | 5/2019-5/2020 |
| Nick Nicoletti | 5/2018-5/2019 |
| Michael Fowler | 5/2016-5/2017 |
| Ana Rubakovic | 5/2016-5/2017 |
| Carl Holland | 5/2014-5/2016 |
| Alexander Hsu | 5/2014-5/2016 |
| Joshua Gilman | 5/2013-5/2015 |
| Jingyi Wang | 5/2013-5/2015 |
Recent Publications
Contact Us
Luebke Lab
650 Albany Street, X317
Boston Massachusetts, 02118
Phone: 617-3588-7725
Email: jluebke@bu.edu