Protein that Promotes Advancement of Prostate Cancer Identified
Researchers at Boston University School of Medicine (BUSM) have discovered that blocking a specific protein, may be a promising strategy to prevent the spread of castration-resistant prostate cancer (CRPC).
Under the direction of BUSM’s Gerald V. Denis, PhD, researchers have long studied a family of three closely related proteins, called BET bromodomain proteins, composed of BRD2, BRD3 and BRD4, which regulate gene expression. BUSM researchers were the first (in the 1990s) to show how these proteins function in human cancer.
These researchers now have discovered that inhibition of the protein BRD4, but not BRD2 or BRD3, consistently regulated prostate cancer cell migration and invasion.
CRPC is a highly aggressive form of prostate cancer that often leads to the development of lethal metastases. Standard of care treatment for patients with CRPC typically includes a means to disrupt androgen receptor (AR) signaling, and while effective for an average of two-three years, treatment inevitably fails to impede progression due to acquired resistance mechanisms to the AR.
“Our findings are significant because current therapeutic options for CRPC are limited and focus primarily on suppressing prostate tumor cells that rely on AR signaling,” explained first author Jordan Shafran, a PhD candidate in the department of molecular and translational medicine at BUSM.
CRPC is a complex, heterogenous disease, with varying AR states and expression patterns across individual tumor cells. As the disease progresses, prostate tumor cells can become less reliant on AR signaling and use alternative signaling mechanisms to sustain growth and dissemination. “Therefore, it is imperative to identify ‘druggable’ targets that regulate prostate cancer cell migration and invasion in cells that are either reliant on, or independent of, androgen receptor signaling,” he added.
These findings appear online in the journal Molecular Cancer Research.
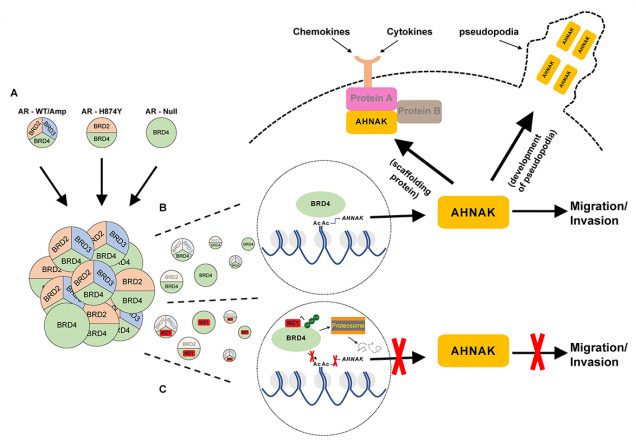
A, Graphical depiction of the different AR statuses represented in the CRPC cell lines from this study, and the corresponding functional BET proteins pertaining to regulating cell migration. The merging of the three cell types and the numbers that represent each type illustrate the AR heterogeneity that has been previously observed in multiple genomic studies of mCRPC tumors.
B, Graphic highlights BRD4 regulation of AHNAK transcription through its interaction with the AHNAK promoter, and how within each cell type, only BRD4 is capable of carrying out this process. AHNAK is then shown to facilitate migration and invasion through its ability to develop pseudopodia while also serving as a scaffolding protein for a variety of canonical signaling pathways that interact with factors secreted from the tumor microenvironment.
C, Illustration showing MZ1 selectively binding too and degrading BRD4 through polyubiquitination and proteasome-dependent degradation. As a result, BRD4 is unable to recruit co-activator proteins to the AHNAK promoter and carry out transcription of AHNAK. Lack of AHNAK represses the cells migratory and invasive capabilities and as a result cell migration and invasion are inhibited.