The insufficient mechanistic information on metastasis has precluded the development of efficient therapeutics for it. The Gα-Interacting, Vesicle-associtated (GIV) protein is emerging as a very promising candidate to become one of the “master regulators” of metastasis and as such, its characterization may open new avenues for therapeutic intervention.
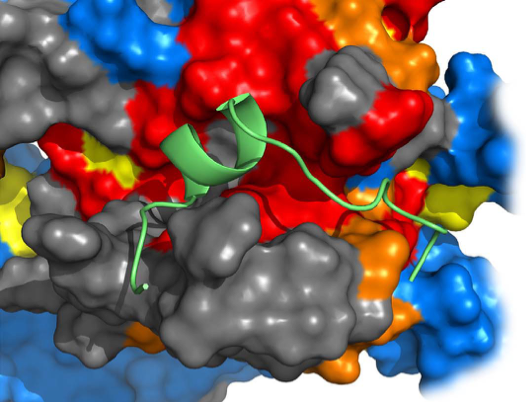
By a combination of biochemical and structural techniques, including NMR, a team of researchers have uncovered the molecular mechanism behind GIV binding and activation of a G protein. G proteins are components of the communication system the body uses to sense hormones in the bloodstream and send the corresponding messages to cells.
The results show that the mode of action of GIV differs from the well known GPCR proteins, because it binds to a different region. Molecular modelling and NMR data inform about the protein-protein interface and show that GIV binds to a cavity on the surface of the G protein. These results suggest and allosteric regulation mechanism as conformational changes in one site propagate to another distant site in the molecule.
The work has been the result of a close collaboration between the group of Mikel García-Marcos at Boston University, and the group of Francisco J Blanco at CIC bioGUNE, and has appeared in the journal Nature Communications.
The synergy between the two teams, and the participation or researchers from IRB Barcelona, Cornell University, and University of Glasgow has made it possible to uncover this novel mode of action of a G protein regulator. Multidisciplinary studies of this kind are key to characterize the complex biological processes relevant in biomedical cancer research.
Reference: Molecular mechanism of Gαi activation by non-GPCR proteins with a Gα-Binding and Activating motif. A Ibáñez de Opakua, K Parag-Sharma, V DiGiacomo, N Merino, A Leyme, A Marivin, M Villate, LT Nguyen, MA de la Cruz-Morcillo, JB Blanco-Canosa, S Ramachandran, George S Baillie, RA Cerione, FJ Blanco, M Garcia-Marcos (2017) Nature Commun 8, 13935.